

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50400098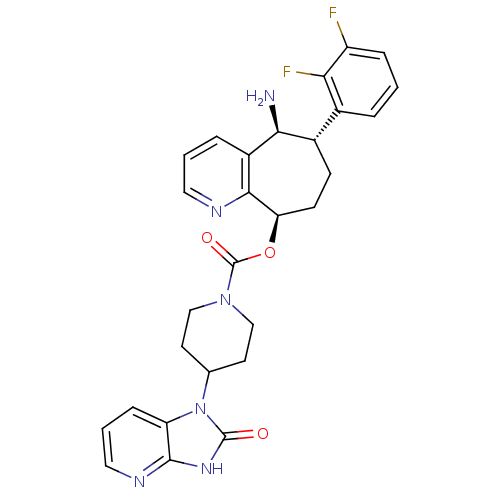 (CHEMBL2178422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50400099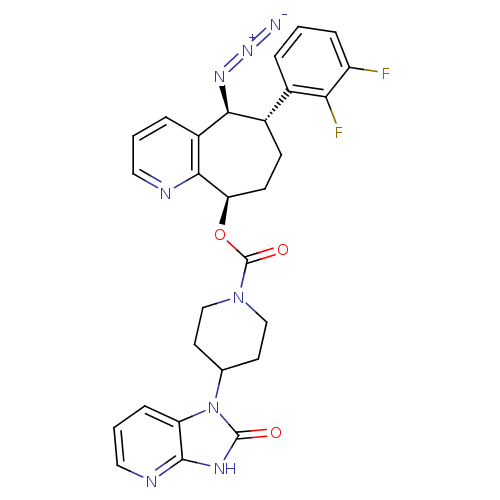 (CHEMBL2178420) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50400102 (CHEMBL2178424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50400100 (CHEMBL2178421) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50400101 (CHEMBL2178423) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037287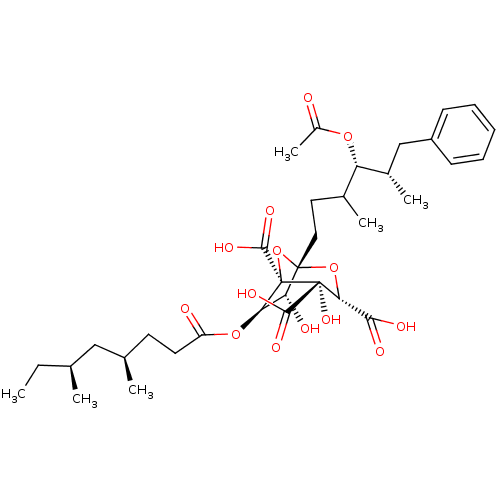 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037286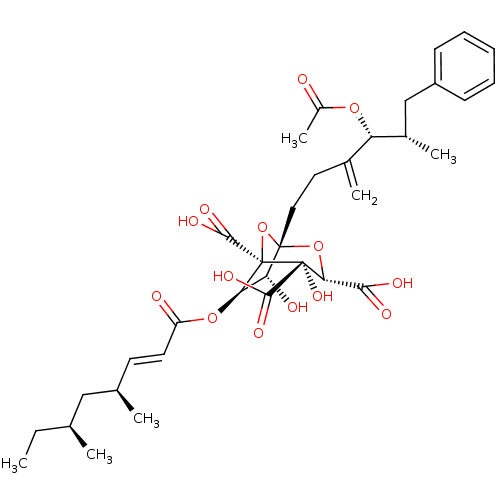 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037285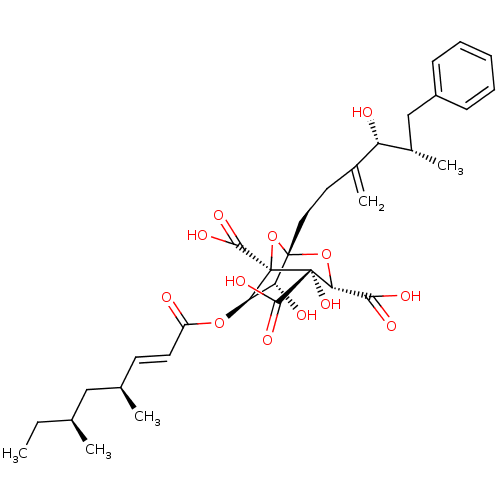 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037279 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037279 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037286 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037289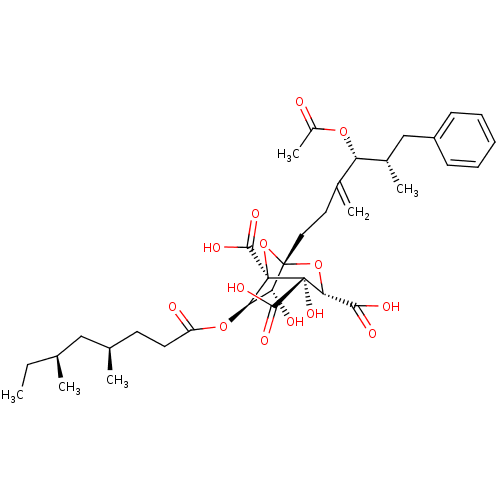 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037291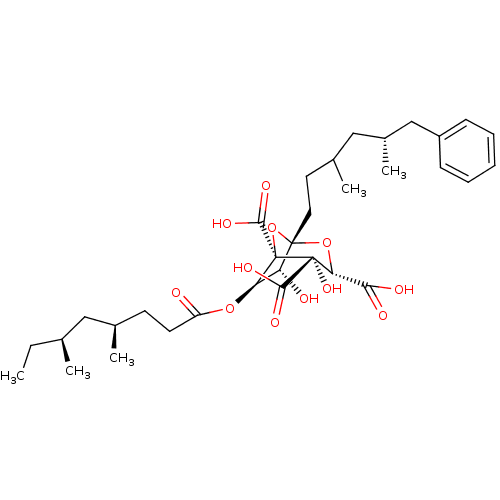 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037291 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037290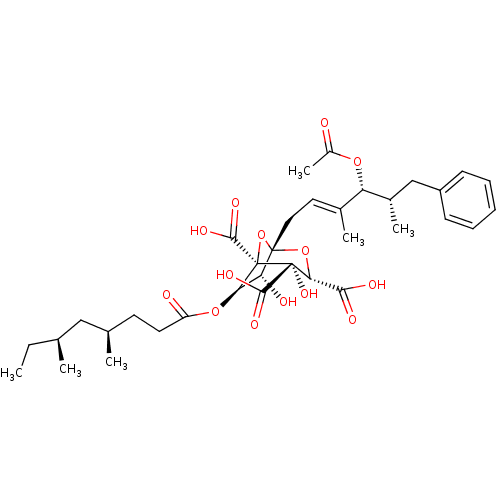 ((1S,3S,4S,5R,6R,7R)-1-((E)-(4R,5S)-4-Acetoxy-3,5-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037280 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037288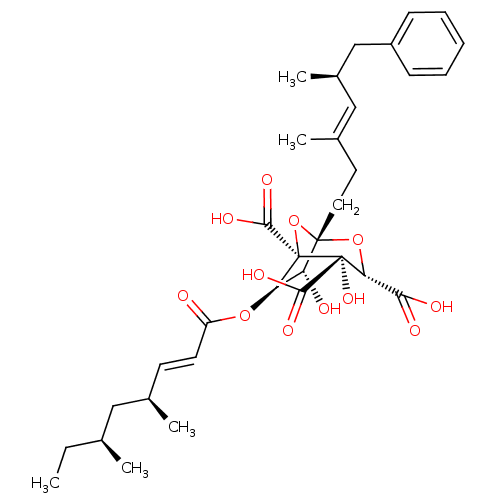 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037282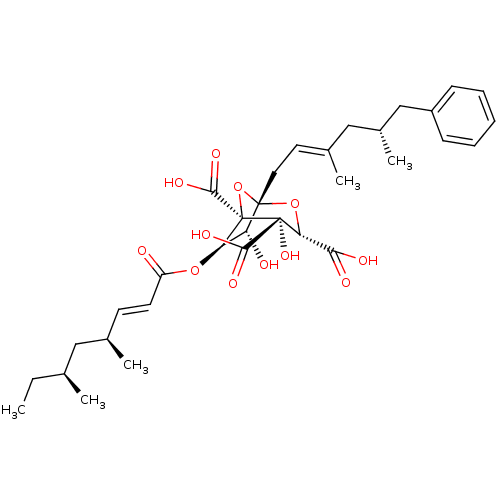 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037284 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037281 ((1S,3S,4S,5R,6R,7R)-1-((R)-3,5-Dimethyl-6-phenyl-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037283 ((1S,3S,4S,5R,6R,7R)-1-((E)-(S)-3,5-Dimethyl-6-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037284 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037283 ((1S,3S,4S,5R,6R,7R)-1-((E)-(S)-3,5-Dimethyl-6-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037292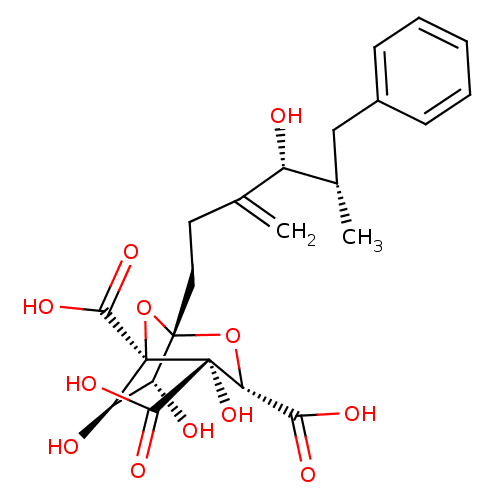 ((1S,3S,4S,5R,6R,7R)-4,6,7-Trihydroxy-1-((4R,5S)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037281 ((1S,3S,4S,5R,6R,7R)-1-((R)-3,5-Dimethyl-6-phenyl-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50400098 (CHEMBL2178422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 using 7-benzyloxy-4-trifluoromethylcoumarin as substrate after 20 mins by fluorescence assay | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50400098 (CHEMBL2178422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 using benzoylresorufin as substrate after 45 mins by fluorescence assay | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50283774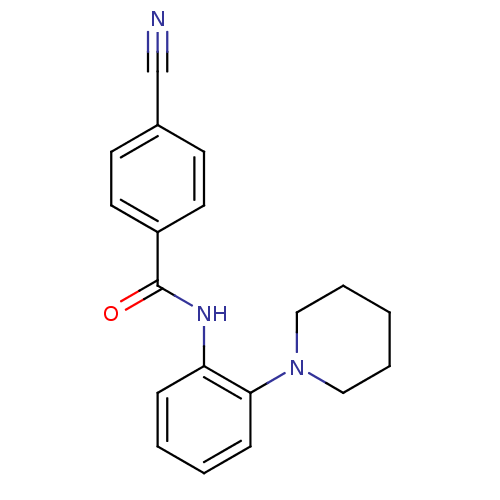 (4-Cyano-N-(2-piperidin-1-yl-phenyl)-benzamide | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP-I | Bioorg Med Chem Lett 4: 2821-2824 (1994) Article DOI: 10.1016/S0960-894X(01)80820-9 BindingDB Entry DOI: 10.7270/Q2S182FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50283773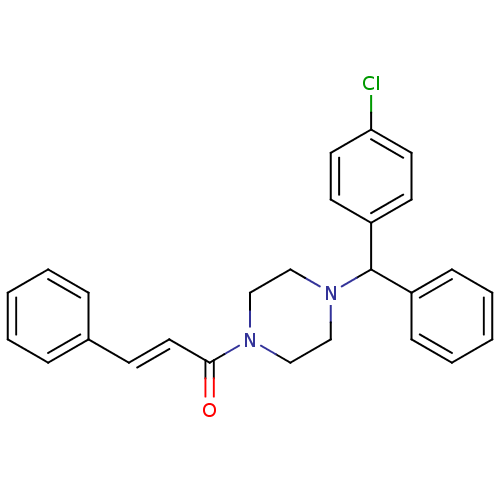 ((E)-1-{4-[(4-Chloro-phenyl)-phenyl-methyl]-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]-senktide binding to human Tachykinin receptor 3 expressed in CHO cells | Bioorg Med Chem Lett 4: 2821-2824 (1994) Article DOI: 10.1016/S0960-894X(01)80820-9 BindingDB Entry DOI: 10.7270/Q2S182FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50400098 (CHEMBL2178422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Antagonist activity at CGRP receptor in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production preincubated for 15 mins prior ... | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||