

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243396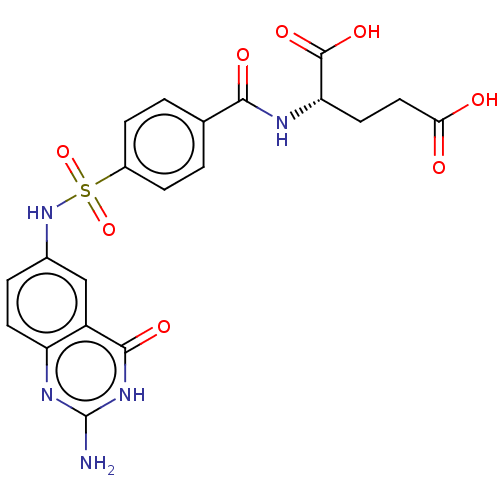 (CHEMBL1231520) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human AICARFT | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human MMP13 using Mca-PQG1 peptide substrate assessed as substrate cleavage after 2 to 4 hrs | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS-4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM194638 (US9206139, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human MMP13 using Mca-PQGL-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-AR-OH as substrate after 2 to 4 hrs by fluorescence assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM194638 (US9206139, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS-5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Competitive inhibition against rat cytoplasmic thymidine kinase | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform A of Ketohexokinase (Peripheral) (Homo sapiens (Human)) | BDBM518444 ((2S,3R)-2-Methyl-1-[4-[1-(1-methylazetidin-3-yl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM194639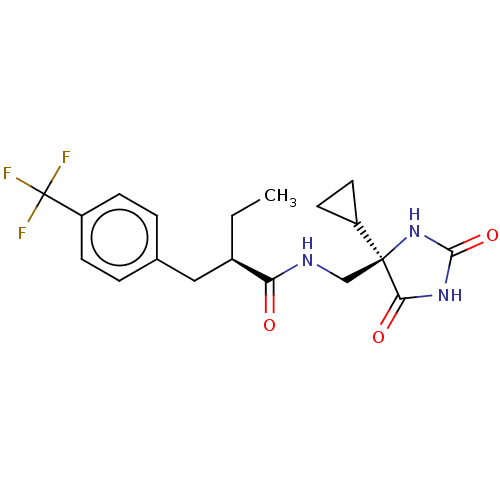 (US9206139, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM194639 (US9206139, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM194644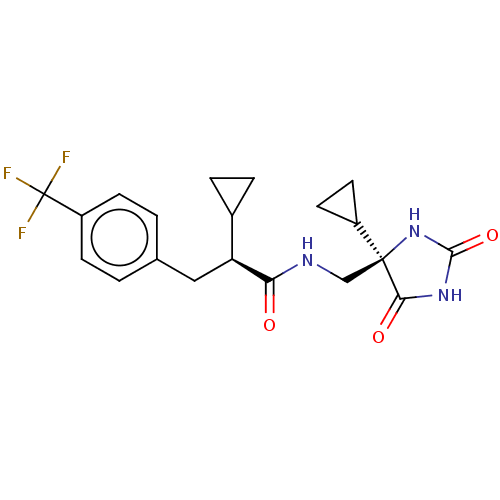 (US9206139, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM194644 (US9206139, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM518444 ((2S,3R)-2-Methyl-1-[4-[1-(1-methylazetidin-3-yl)py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50532313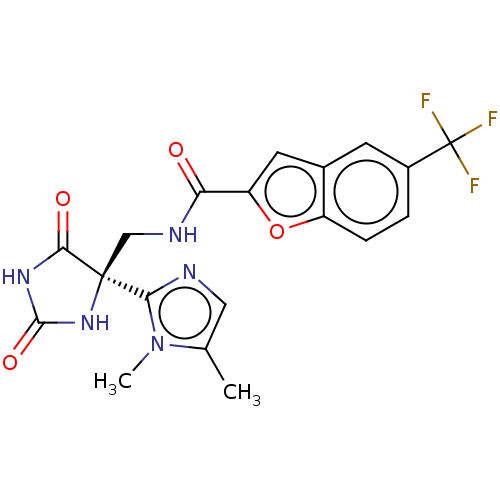 (CHEMBL4436740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human MMP3 using Mca-PQG1 peptide substrate assessed as substrate cleavage after 2 to 4 hrs | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50532313 (CHEMBL4436740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50033806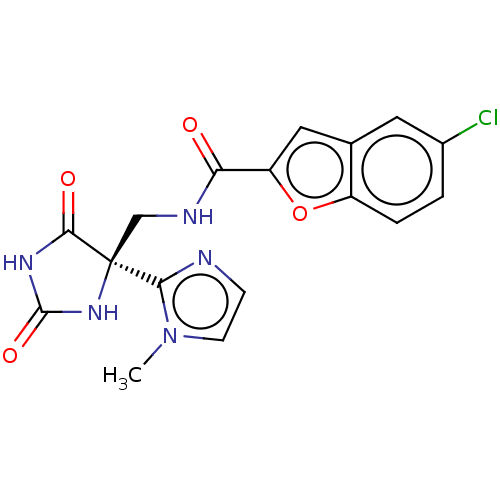 (CHEMBL3358156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50033806 (CHEMBL3358156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50033806 (CHEMBL3358156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human MMP3 using Mca-PQGL-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-AR-OH as substrate after 2 to 4 hrs by fluorescence assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50033806 (CHEMBL3358156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform A of Ketohexokinase (Peripheral) (Homo sapiens (Human)) | BDBM518415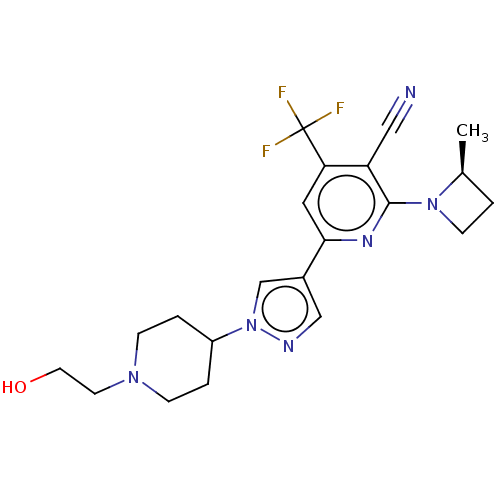 (6-[1-[1-(2-Hydroxyethyl)-4-piperidyl]pyrazol-4-yl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform A of Ketohexokinase (Peripheral) (Homo sapiens (Human)) | BDBM518442 (4-[1-(Azetidin-3-yl)pyrazol-4-yl]-2-[(2S)-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform A of Ketohexokinase (Peripheral) (Homo sapiens (Human)) | BDBM518418 (2-[(2S)-2-Methylazetidin-1-yl]-6-[1-(4-piperidyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM518443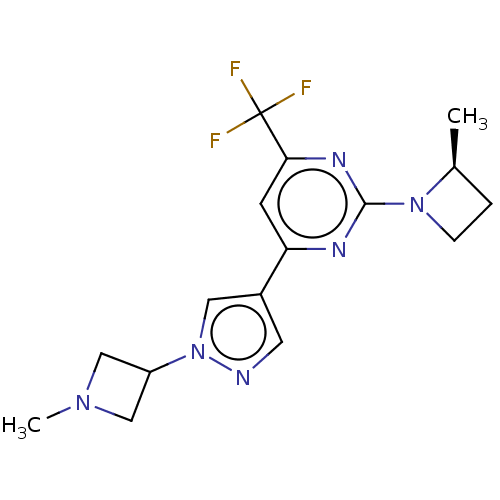 (2-[(2S)-2-Methylazetidin-1-yl]-4-[1-(1-methylazeti...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM518418 (2-[(2S)-2-Methylazetidin-1-yl]-6-[1-(4-piperidyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform A of Ketohexokinase (Peripheral) (Homo sapiens (Human)) | BDBM518445 (US11124500, Example 36 | [(2R)-1-[4-[1-(1-Methylaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50532312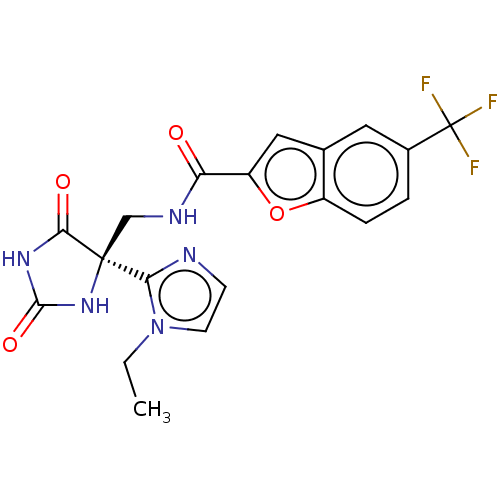 (CHEMBL4450729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50033808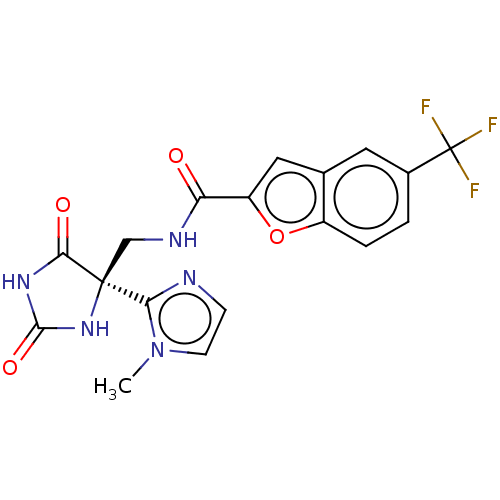 (CHEMBL3358158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM194646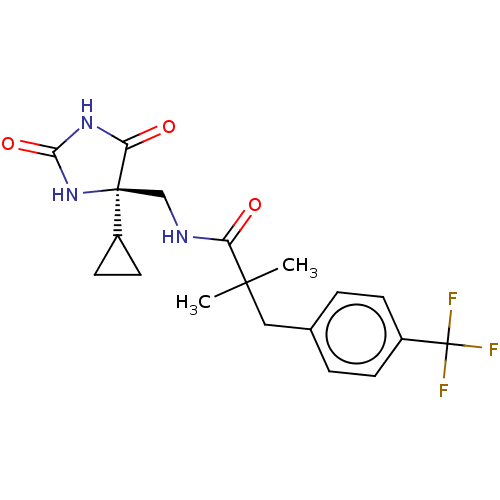 (US9206139, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50238241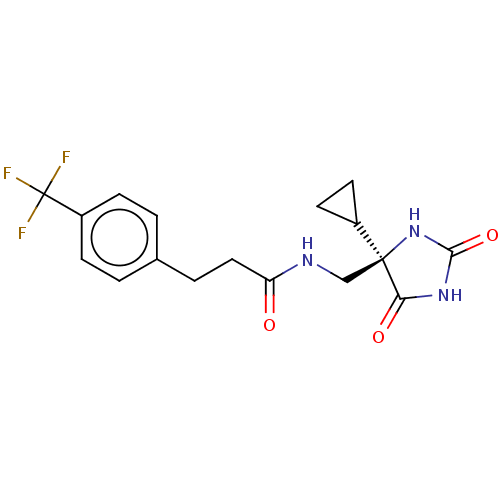 (CHEMBL4102193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay | J Med Chem 60: 5933-5939 (2017) Article DOI: 10.1021/acs.jmedchem.7b00650 BindingDB Entry DOI: 10.7270/Q2Z321XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50033808 (CHEMBL3358158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS-5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50033808 (CHEMBL3358158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS-4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50033808 (CHEMBL3358158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50532312 (CHEMBL4450729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50532313 (CHEMBL4436740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50532313 (CHEMBL4436740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50532312 (CHEMBL4450729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50033808 (CHEMBL3358158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50532312 (CHEMBL4450729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50033808 (CHEMBL3358158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform A of Ketohexokinase (Peripheral) (Homo sapiens (Human)) | BDBM518435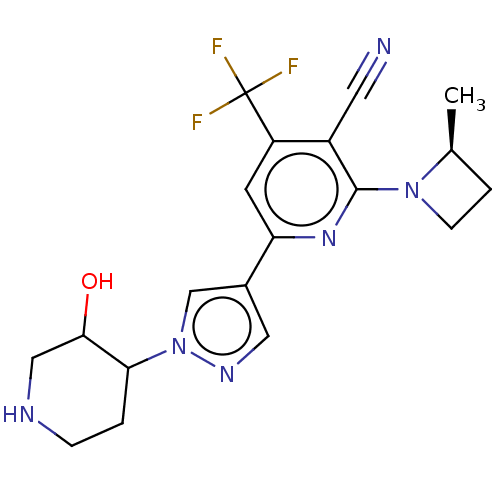 (6-[1-(3-Hydroxy-4-piperidyl)pyrazol-4-yl]-2-[(2S)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM518432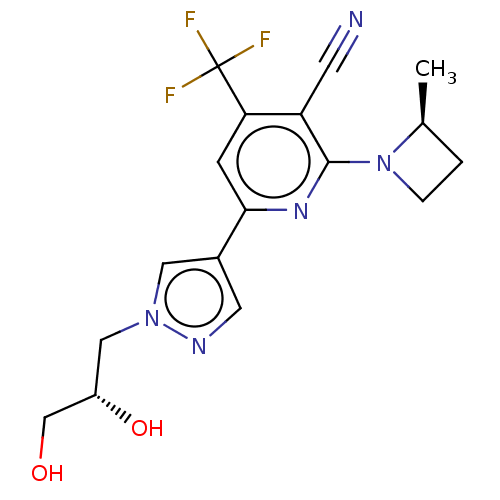 (US11124500, Example 24b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM518442 (4-[1-(Azetidin-3-yl)pyrazol-4-yl]-2-[(2S)-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human MMP2 using Mca-PQG1 peptide substrate assessed as substrate cleavage after 2 to 4 hrs | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50033806 (CHEMBL3358156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS-4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50238243 (CHEMBL4097165) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay | J Med Chem 59: 5810-22 (2016) Article DOI: 10.1021/acs.jmedchem.6b00398 BindingDB Entry DOI: 10.7270/Q280563T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 411 total ) | Next | Last >> |