 Found 206 hits with Last Name = 'jiménez' and Initial = 'jm'
Found 206 hits with Last Name = 'jiménez' and Initial = 'jm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150210
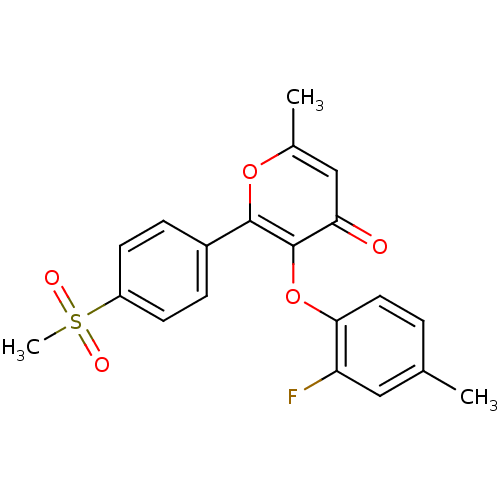
(3-(2-Fluoro-4-methyl-phenoxy)-2-(4-methanesulfonyl...)Show SMILES Cc1ccc(Oc2c(oc(C)cc2=O)-c2ccc(cc2)S(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C20H17FO5S/c1-12-4-9-18(16(21)10-12)26-20-17(22)11-13(2)25-19(20)14-5-7-15(8-6-14)27(3,23)24/h4-11H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150211

(3-(2,4-Difluoro-phenoxy)-2-(4-methanesulfonyl-phen...)Show SMILES Cc1cc(=O)c(Oc2ccc(F)cc2F)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H14F2O5S/c1-11-9-16(22)19(26-17-8-5-13(20)10-15(17)21)18(25-11)12-3-6-14(7-4-12)27(2,23)24/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150226
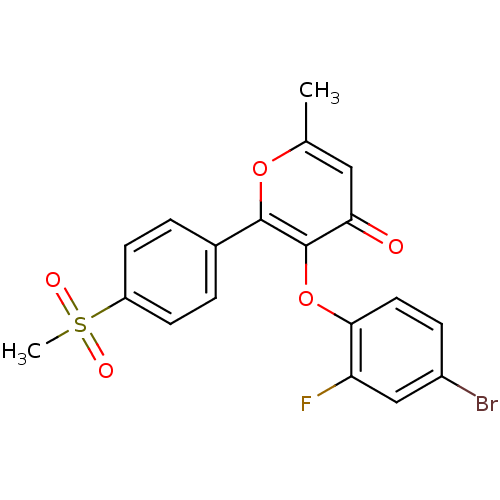
(3-(4-Bromo-2-fluoro-phenoxy)-2-(4-methanesulfonyl-...)Show SMILES Cc1cc(=O)c(Oc2ccc(Br)cc2F)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H14BrFO5S/c1-11-9-16(22)19(26-17-8-5-13(20)10-15(17)21)18(25-11)12-3-6-14(7-4-12)27(2,23)24/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150202

(2-(4-Methanesulfonyl-phenyl)-6-methyl-3-o-tolyloxy...)Show SMILES Cc1cc(=O)c(Oc2ccccc2C)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H18O5S/c1-13-6-4-5-7-18(13)25-20-17(21)12-14(2)24-19(20)15-8-10-16(11-9-15)26(3,22)23/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150215

(3-(4-Fluoro-2-methyl-phenoxy)-2-(4-methanesulfonyl...)Show SMILES Cc1cc(=O)c(Oc2ccc(F)cc2C)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H17FO5S/c1-12-10-15(21)6-9-18(12)26-20-17(22)11-13(2)25-19(20)14-4-7-16(8-5-14)27(3,23)24/h4-11H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150193
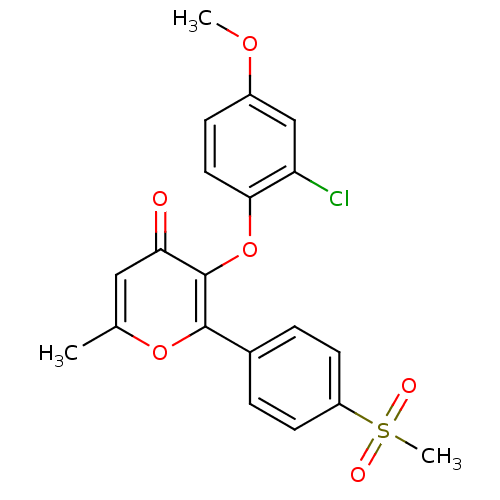
(3-(2-Chloro-4-methoxy-phenoxy)-2-(4-methanesulfony...)Show SMILES COc1ccc(Oc2c(oc(C)cc2=O)-c2ccc(cc2)S(C)(=O)=O)c(Cl)c1 Show InChI InChI=1S/C20H17ClO6S/c1-12-10-17(22)20(27-18-9-6-14(25-2)11-16(18)21)19(26-12)13-4-7-15(8-5-13)28(3,23)24/h4-11H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150197

(3-(4-Chloro-2-methyl-phenoxy)-2-(4-methanesulfonyl...)Show SMILES Cc1cc(=O)c(Oc2ccc(Cl)cc2C)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H17ClO5S/c1-12-10-15(21)6-9-18(12)26-20-17(22)11-13(2)25-19(20)14-4-7-16(8-5-14)27(3,23)24/h4-11H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150185
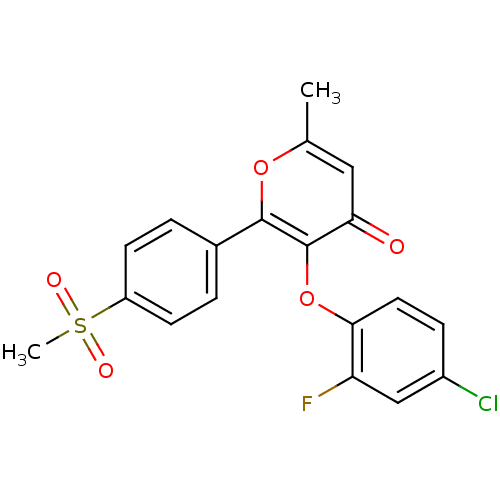
(3-(4-Chloro-2-fluoro-phenoxy)-2-(4-methanesulfonyl...)Show SMILES Cc1cc(=O)c(Oc2ccc(Cl)cc2F)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H14ClFO5S/c1-11-9-16(22)19(26-17-8-5-13(20)10-15(17)21)18(25-11)12-3-6-14(7-4-12)27(2,23)24/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084355
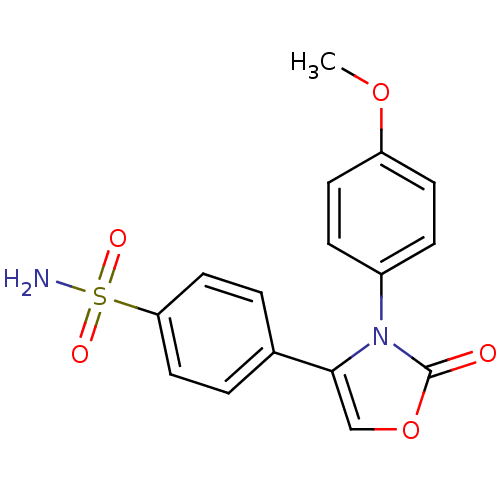
(4-[3-(4-Methoxy-phenyl)-2-oxo-2,3-dihydro-oxazol-4...)Show SMILES COc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O5S/c1-22-13-6-4-12(5-7-13)18-15(10-23-16(18)19)11-2-8-14(9-3-11)24(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150224

(2-(4-Methanesulfonyl-phenyl)-6-methyl-3-p-tolyloxy...)Show SMILES Cc1ccc(Oc2c(oc(C)cc2=O)-c2ccc(cc2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C20H18O5S/c1-13-4-8-16(9-5-13)25-20-18(21)12-14(2)24-19(20)15-6-10-17(11-7-15)26(3,22)23/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105290

(2-[7-Methoxy-4-(4-methyl-benzyl)-naphthalen-1-yl]-...)Show InChI InChI=1S/C22H22O3/c1-14-4-6-16(7-5-14)12-17-8-10-19(15(2)22(23)24)21-13-18(25-3)9-11-20(17)21/h4-11,13,15H,12H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084352

(4-[3-(3,4-Dichloro-phenyl)-5-methyl-2-oxo-2,3-dihy...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C16H12Cl2N2O4S/c1-9-15(10-2-5-12(6-3-10)25(19,22)23)20(16(21)24-9)11-4-7-13(17)14(18)8-11/h2-8H,1H3,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150225
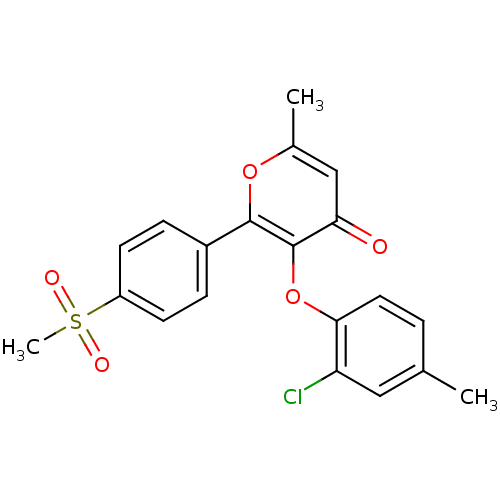
(3-(2-Chloro-4-methyl-phenoxy)-2-(4-methanesulfonyl...)Show SMILES Cc1ccc(Oc2c(oc(C)cc2=O)-c2ccc(cc2)S(C)(=O)=O)c(Cl)c1 Show InChI InChI=1S/C20H17ClO5S/c1-12-4-9-18(16(21)10-12)26-20-17(22)11-13(2)25-19(20)14-5-7-15(8-6-14)27(3,23)24/h4-11H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150198
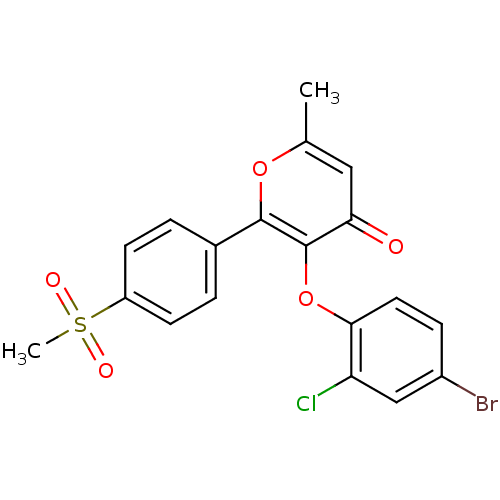
(3-(4-Bromo-2-chloro-phenoxy)-2-(4-methanesulfonyl-...)Show SMILES Cc1cc(=O)c(Oc2ccc(Br)cc2Cl)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H14BrClO5S/c1-11-9-16(22)19(26-17-8-5-13(20)10-15(17)21)18(25-11)12-3-6-14(7-4-12)27(2,23)24/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084366
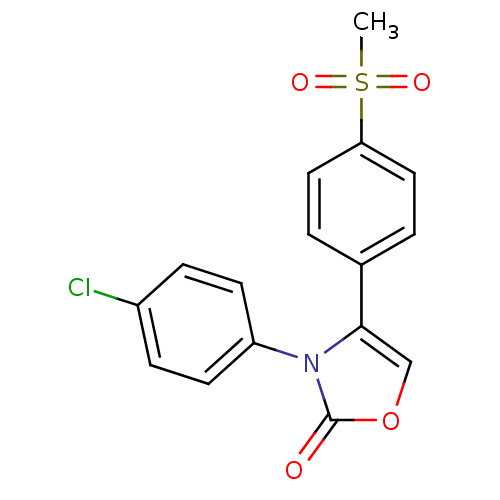
(3-(4-Chloro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C16H12ClNO4S/c1-23(20,21)14-8-2-11(3-9-14)15-10-22-16(19)18(15)13-6-4-12(17)5-7-13/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150194
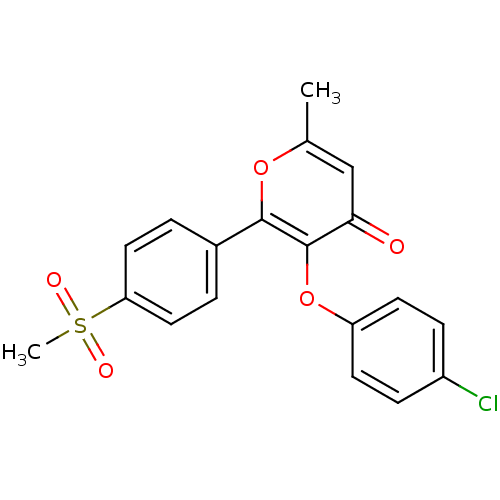
(3-(4-Chloro-phenoxy)-2-(4-methanesulfonyl-phenyl)-...)Show SMILES Cc1cc(=O)c(Oc2ccc(Cl)cc2)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H15ClO5S/c1-12-11-17(21)19(25-15-7-5-14(20)6-8-15)18(24-12)13-3-9-16(10-4-13)26(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105302
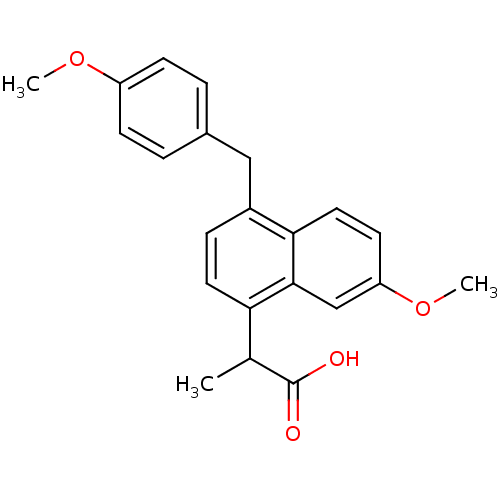
(2-[7-Methoxy-4-(4-methoxy-benzyl)-naphthalen-1-yl]...)Show SMILES COc1ccc(Cc2ccc(C(C)C(O)=O)c3cc(OC)ccc23)cc1 Show InChI InChI=1S/C22H22O4/c1-14(22(23)24)19-10-6-16(12-15-4-7-17(25-2)8-5-15)20-11-9-18(26-3)13-21(19)20/h4-11,13-14H,12H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150222

(3-(4-Bromo-phenoxy)-2-(4-methanesulfonyl-phenyl)-6...)Show SMILES Cc1cc(=O)c(Oc2ccc(Br)cc2)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H15BrO5S/c1-12-11-17(21)19(25-15-7-5-14(20)6-8-15)18(24-12)13-3-9-16(10-4-13)26(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105293
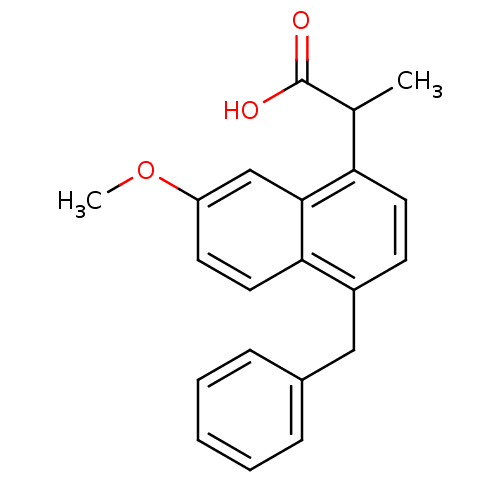
(2-(4-Benzyl-7-methoxy-naphthalen-1-yl)-propionic a...)Show InChI InChI=1S/C21H20O3/c1-14(21(22)23)18-10-8-16(12-15-6-4-3-5-7-15)19-11-9-17(24-2)13-20(18)19/h3-11,13-14H,12H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084359
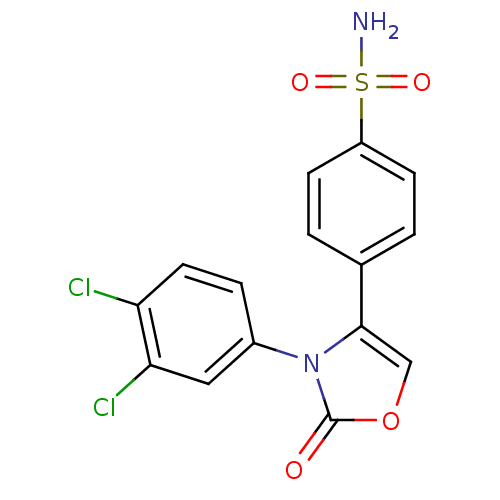
(4-[3-(3,4-Dichloro-phenyl)-2-oxo-2,3-dihydro-oxazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H10Cl2N2O4S/c16-12-6-3-10(7-13(12)17)19-14(8-23-15(19)20)9-1-4-11(5-2-9)24(18,21)22/h1-8H,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150212
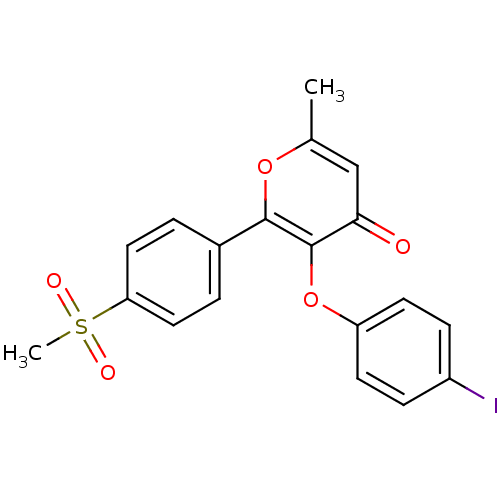
(3-(4-Iodo-phenoxy)-2-(4-methanesulfonyl-phenyl)-6-...)Show SMILES Cc1cc(=O)c(Oc2ccc(I)cc2)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H15IO5S/c1-12-11-17(21)19(25-15-7-5-14(20)6-8-15)18(24-12)13-3-9-16(10-4-13)26(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084345
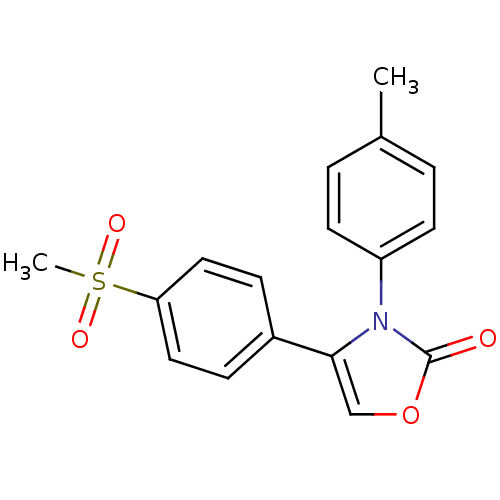
(4-(4-Methanesulfonyl-phenyl)-3-p-tolyl-3H-oxazol-2...)Show SMILES Cc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C17H15NO4S/c1-12-3-7-14(8-4-12)18-16(11-22-17(18)19)13-5-9-15(10-6-13)23(2,20)21/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084368
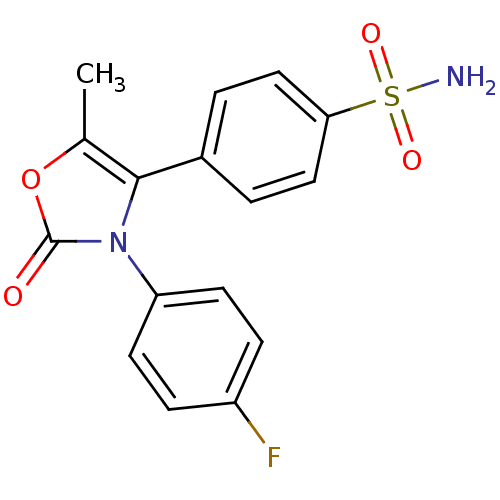
(4-[3-(4-Fluoro-phenyl)-5-methyl-2-oxo-2,3-dihydro-...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C16H13FN2O4S/c1-10-15(11-2-8-14(9-3-11)24(18,21)22)19(16(20)23-10)13-6-4-12(17)5-7-13/h2-9H,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084353
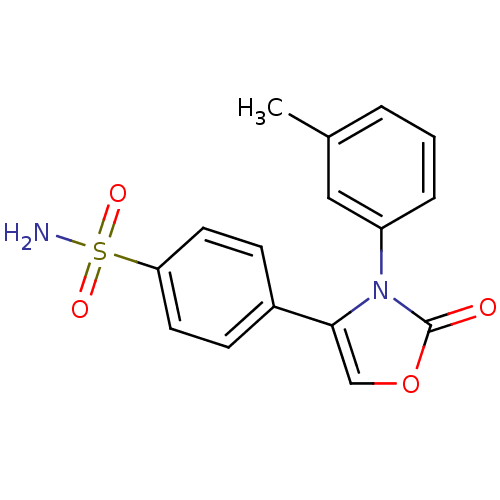
(4-(2-Oxo-3-m-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1cccc(c1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O4S/c1-11-3-2-4-13(9-11)18-15(10-22-16(18)19)12-5-7-14(8-6-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084347
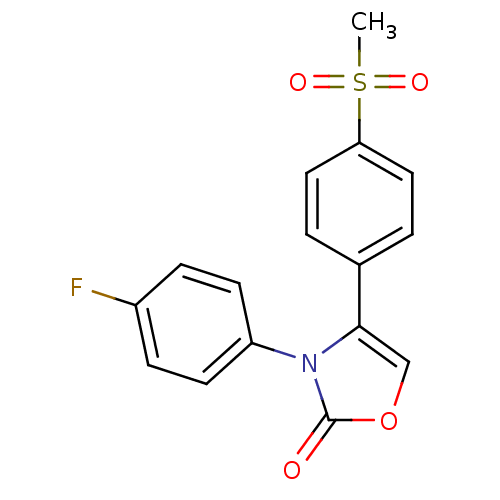
(3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C16H12FNO4S/c1-23(20,21)14-8-2-11(3-9-14)15-10-22-16(19)18(15)13-6-4-12(17)5-7-13/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105303
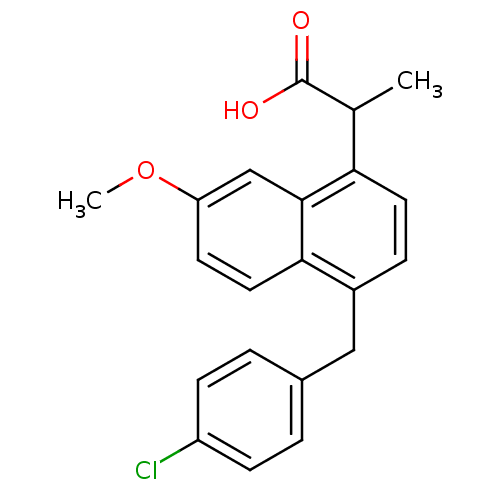
(2-[4-(4-Chloro-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show SMILES COc1ccc2c(Cc3ccc(Cl)cc3)ccc(C(C)C(O)=O)c2c1 Show InChI InChI=1S/C21H19ClO3/c1-13(21(23)24)18-9-5-15(11-14-3-6-16(22)7-4-14)19-10-8-17(25-2)12-20(18)19/h3-10,12-13H,11H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084349
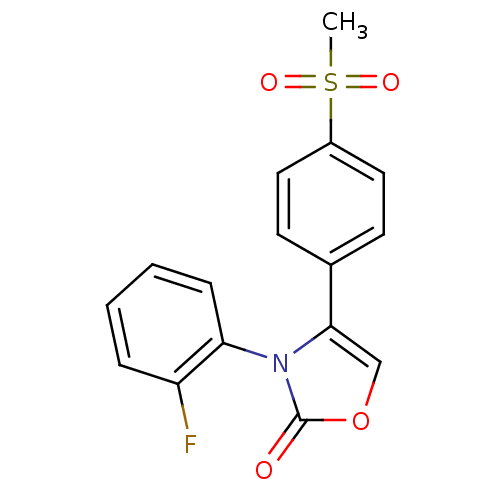
(3-(2-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccccc1F |(5.04,-8.06,;6.08,-9.19,;7.18,-10.29,;5.08,-10.45,;7.11,-8.04,;6.63,-6.59,;7.67,-5.45,;9.16,-5.76,;9.64,-7.22,;8.62,-8.36,;10.18,-4.61,;11.71,-4.76,;12.32,-3.35,;11.18,-2.33,;11.34,-.8,;9.85,-3.1,;8.76,-2.01,;9.16,-.51,;8.06,.6,;6.55,.19,;6.15,-1.33,;7.26,-2.42,;6.87,-3.89,)| Show InChI InChI=1S/C16H12FNO4S/c1-23(20,21)12-8-6-11(7-9-12)15-10-22-16(19)18(15)14-5-3-2-4-13(14)17/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150214
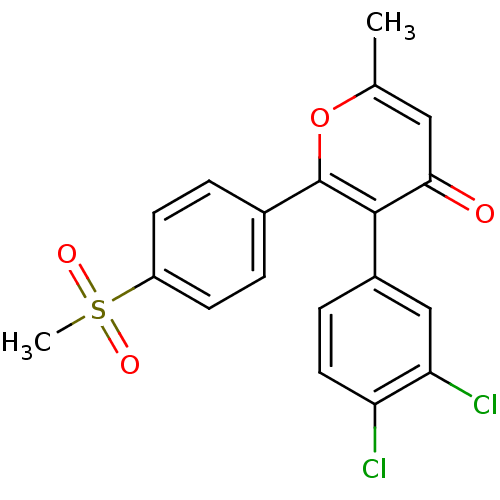
(3-(3,4-Dichloro-phenyl)-2-(4-methanesulfonyl-pheny...)Show SMILES Cc1cc(=O)c(-c2ccc(Cl)c(Cl)c2)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H14Cl2O4S/c1-11-9-17(22)18(13-5-8-15(20)16(21)10-13)19(25-11)12-3-6-14(7-4-12)26(2,23)24/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084346
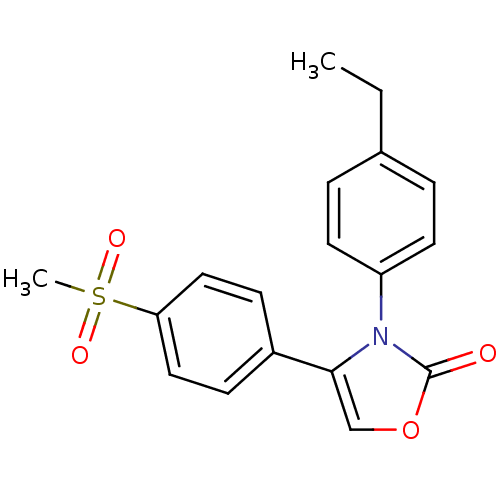
(3-(4-Ethyl-phenyl)-4-(4-methanesulfonyl-phenyl)-3H...)Show SMILES CCc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H17NO4S/c1-3-13-4-8-15(9-5-13)19-17(12-23-18(19)20)14-6-10-16(11-7-14)24(2,21)22/h4-12H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105289
(2-[4-(4-Fluoro-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show InChI InChI=1S/C21H19FO3/c1-13(21(23)24)18-9-5-15(11-14-3-6-16(22)7-4-14)19-10-8-17(25-2)12-20(18)19/h3-10,12-13H,11H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084370

(4-[3-(3-Chloro-4-methoxy-phenyl)-2-oxo-2,3-dihydro...)Show SMILES COc1ccc(cc1Cl)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13ClN2O5S/c1-23-15-7-4-11(8-13(15)17)19-14(9-24-16(19)20)10-2-5-12(6-3-10)25(18,21)22/h2-9H,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084364
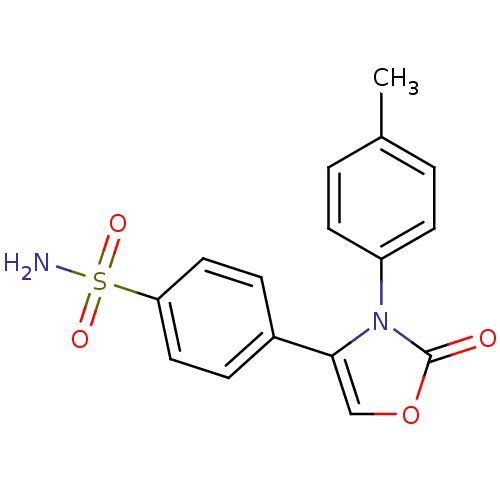
(4-(2-Oxo-3-p-tolyl-2,3-dihydro-oxazol-4-yl)-benzen...)Show SMILES Cc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O4S/c1-11-2-6-13(7-3-11)18-15(10-22-16(18)19)12-4-8-14(9-5-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
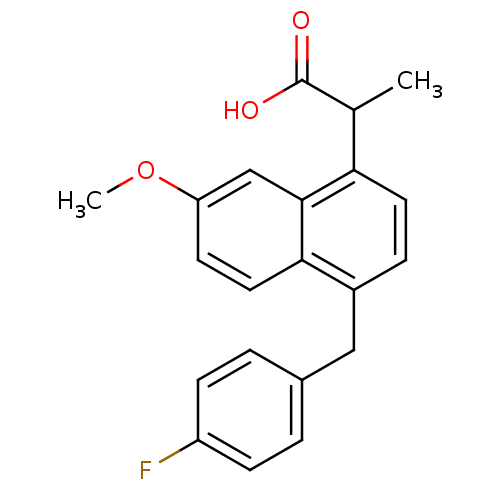
(RAT) | BDBM50105288
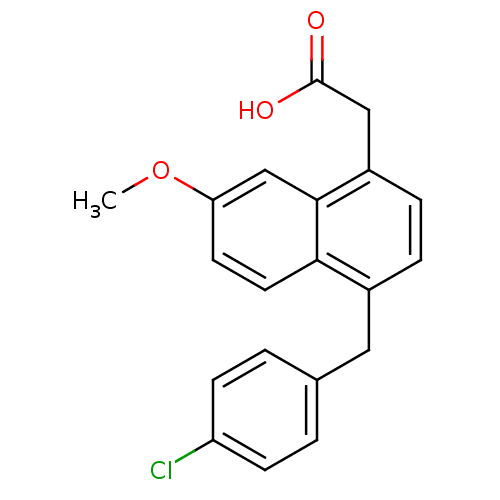
(CHEMBL91139 | [4-(4-Chloro-benzyl)-7-methoxy-napht...)Show InChI InChI=1S/C20H17ClO3/c1-24-17-8-9-18-14(10-13-2-6-16(21)7-3-13)4-5-15(11-20(22)23)19(18)12-17/h2-9,12H,10-11H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084355

(4-[3-(4-Methoxy-phenyl)-2-oxo-2,3-dihydro-oxazol-4...)Show SMILES COc1ccc(cc1)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H14N2O5S/c1-22-13-6-4-12(5-7-13)18-15(10-23-16(18)19)11-2-8-14(9-3-11)24(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084350

(4-(5-Methyl-2-oxo-3-m-tolyl-2,3-dihydro-oxazol-4-y...)Show SMILES Cc1oc(=O)n(c1-c1ccc(cc1)S(N)(=O)=O)-c1cccc(C)c1 Show InChI InChI=1S/C17H16N2O4S/c1-11-4-3-5-14(10-11)19-16(12(2)23-17(19)20)13-6-8-15(9-7-13)24(18,21)22/h3-10H,1-2H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150218
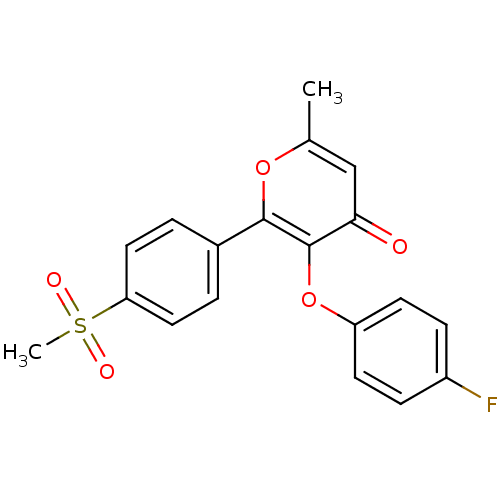
(3-(4-Fluoro-phenoxy)-2-(4-methanesulfonyl-phenyl)-...)Show SMILES Cc1cc(=O)c(Oc2ccc(F)cc2)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H15FO5S/c1-12-11-17(21)19(25-15-7-5-14(20)6-8-15)18(24-12)13-3-9-16(10-4-13)26(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150199
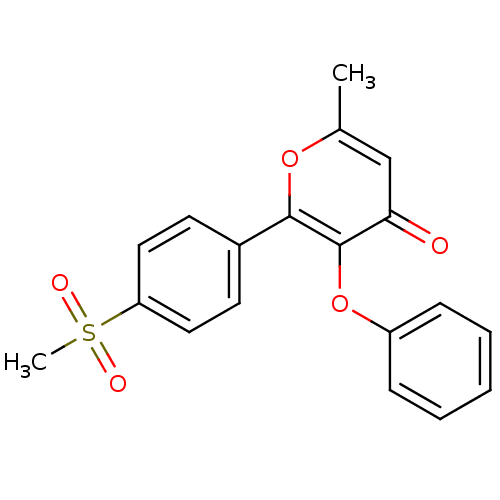
(2-(4-Methanesulfonyl-phenyl)-6-methyl-3-phenoxy-py...)Show SMILES Cc1cc(=O)c(Oc2ccccc2)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H16O5S/c1-13-12-17(20)19(24-15-6-4-3-5-7-15)18(23-13)14-8-10-16(11-9-14)25(2,21)22/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105282
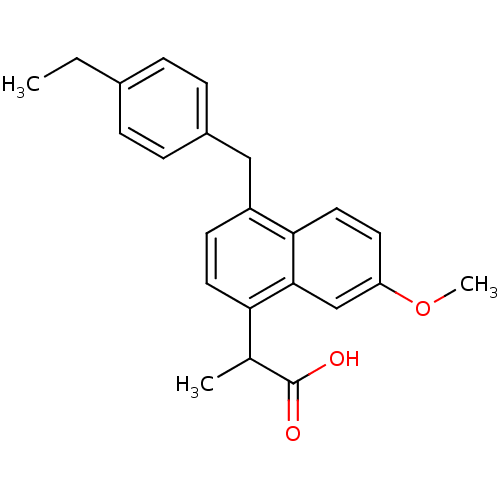
(2-[4-(4-Ethyl-benzyl)-7-methoxy-naphthalen-1-yl]-p...)Show SMILES CCc1ccc(Cc2ccc(C(C)C(O)=O)c3cc(OC)ccc23)cc1 Show InChI InChI=1S/C23H24O3/c1-4-16-5-7-17(8-6-16)13-18-9-11-20(15(2)23(24)25)22-14-19(26-3)10-12-21(18)22/h5-12,14-15H,4,13H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150216
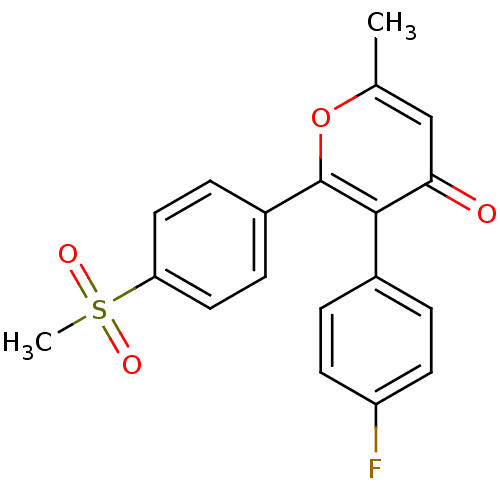
(3-(4-Fluoro-phenyl)-2-(4-methanesulfonyl-phenyl)-6...)Show SMILES Cc1cc(=O)c(-c2ccc(F)cc2)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H15FO4S/c1-12-11-17(21)18(13-3-7-15(20)8-4-13)19(24-12)14-5-9-16(10-6-14)25(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150204

(3-(2,4-Difluoro-phenyl)-2-(4-methanesulfonyl-pheny...)Show SMILES Cc1cc(=O)c(-c2ccc(F)cc2F)c(o1)-c1ccc(cc1)S(C)(=O)=O |(4.05,-1.57,;2.71,-.79,;2.71,.76,;1.37,1.53,;1.37,3.07,;.05,.76,;-1.3,1.53,;-2.62,.76,;-3.96,1.53,;-3.96,3.07,;-5.28,3.83,;-2.62,3.83,;-1.3,3.07,;-.2,4.16,;.05,-.79,;1.37,-1.56,;-1.3,-1.56,;-1.3,-3.1,;-2.62,-3.87,;-3.95,-3.1,;-3.95,-1.56,;-2.62,-.79,;-5.3,-3.89,;-6.61,-4.66,;-6.08,-2.55,;-4.51,-5.22,)| Show InChI InChI=1S/C19H14F2O4S/c1-11-9-17(22)18(15-8-5-13(20)10-16(15)21)19(25-11)12-3-6-14(7-4-12)26(2,23)24/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition concentration against cyclooxygenase-2 (COX-2) in human whole blood |
J Med Chem 47: 3874-86 (2004)
Article DOI: 10.1021/jm049882t
BindingDB Entry DOI: 10.7270/Q2HM57WX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084372
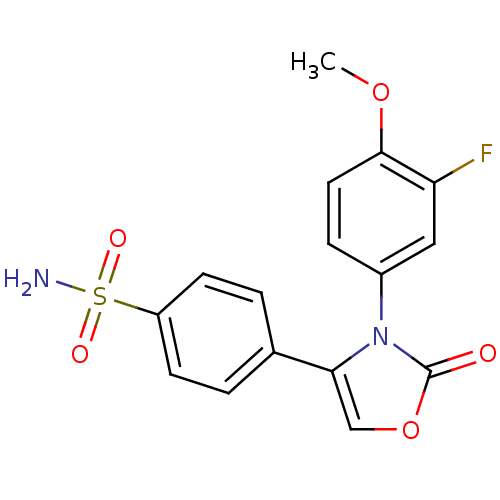
(4-[3-(3-Fluoro-4-methoxy-phenyl)-2-oxo-2,3-dihydro...)Show SMILES COc1ccc(cc1F)-n1c(coc1=O)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13FN2O5S/c1-23-15-7-4-11(8-13(15)17)19-14(9-24-16(19)20)10-2-5-12(6-3-10)25(18,21)22/h2-9H,1H3,(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 |
J Med Chem 43: 214-23 (2000)
BindingDB Entry DOI: 10.7270/Q2DV1KK0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data