

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50026690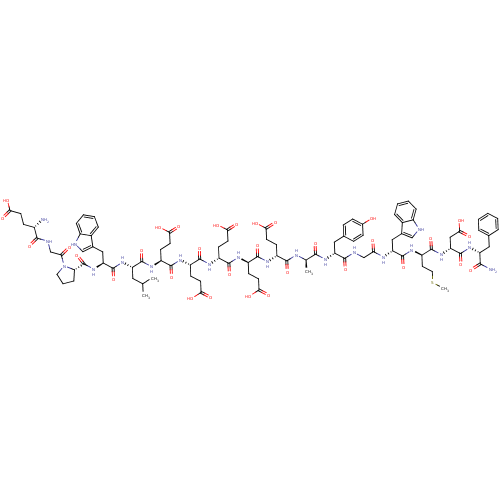 (2-[2-(2-{2-[2-{2-[2-(2-Amino-3-phenyl-propionylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of the binding of [125I]-(Nle11)-HG-13 to Histamine H2 receptor | J Med Chem 27: 1597-601 (1985) BindingDB Entry DOI: 10.7270/Q29P30PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50026688 (3-(2-tert-Butoxycarbonylamino-4-methylsulfanyl-but...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of the binding of [125I]-(Nle11)-HG-13 to Histamine H2 receptor | J Med Chem 27: 1597-601 (1985) BindingDB Entry DOI: 10.7270/Q29P30PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50024328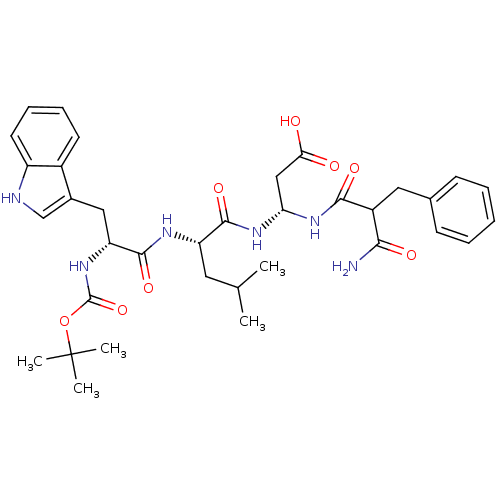 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity for binding of [125I]-(Nle11)-HG-13 to gastrin receptor on isolated rabbit gastric mucosal cells. | J Med Chem 30: 758-63 (1987) BindingDB Entry DOI: 10.7270/Q2CV4GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50024330 (3-(3-Benzyl-ureido)-3-{2-[2-tert-butoxycarbonylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity for binding of [125I]-(Nle11)-HG-13 to gastrin receptor on isolated rabbit gastric mucosal cells. | J Med Chem 30: 758-63 (1987) BindingDB Entry DOI: 10.7270/Q2CV4GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50024330 (3-(3-Benzyl-ureido)-3-{2-[2-tert-butoxycarbonylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020575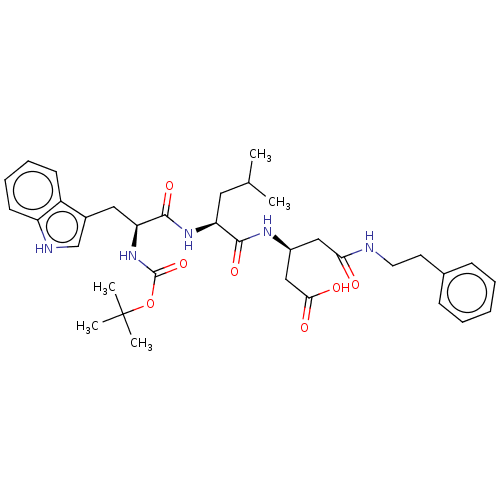 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020560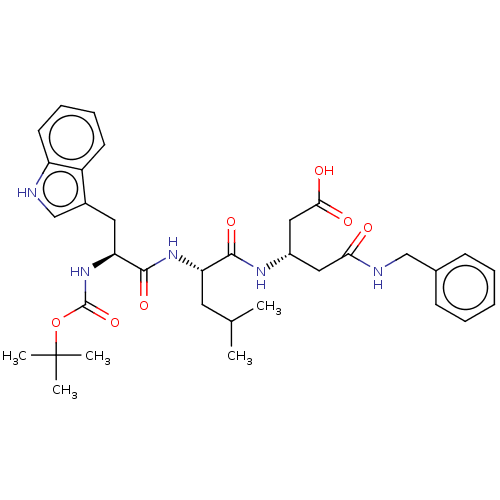 (4-Benzylcarbamoyl-3-{2-[2-tert-butoxycarbonylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50024327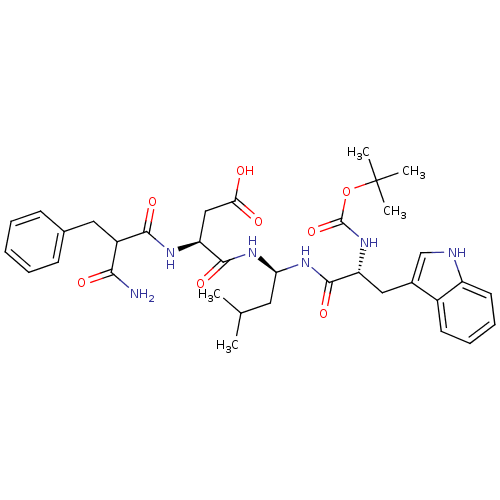 (CHEMBL349888 | N-{1-[2-tert-Butoxycarbonylamino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity for binding of [125I]-(Nle11)-HG-13 to gastrin receptor on isolated rabbit gastric mucosal cells. | J Med Chem 30: 758-63 (1987) BindingDB Entry DOI: 10.7270/Q2CV4GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020564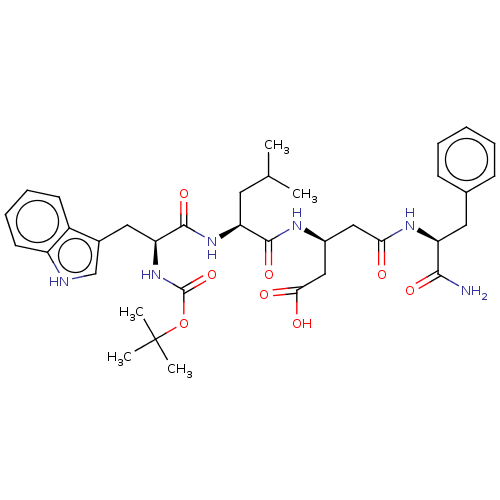 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020569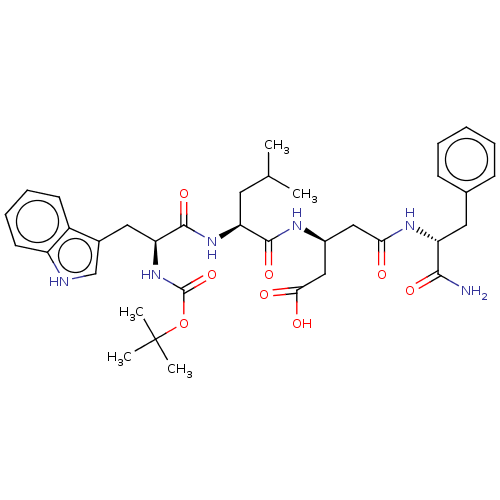 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020561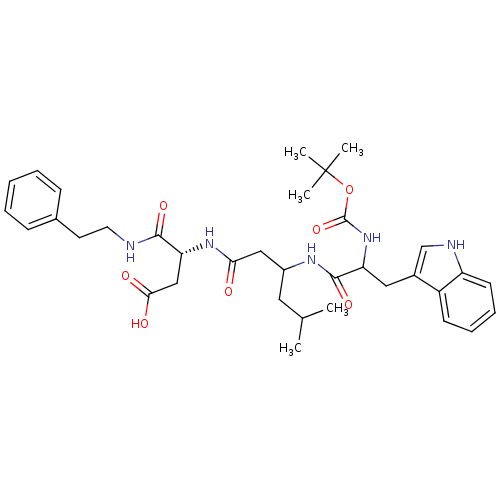 (3-{3-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50026691 (4-Benzyloxycarbonylamino-4-{1-[1-({[1-[1-(1-carbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of the binding of [125I]-(Nle11)-HG-13 to Histamine H2 receptor | J Med Chem 27: 1597-601 (1985) BindingDB Entry DOI: 10.7270/Q29P30PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020573 (3-{3-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020574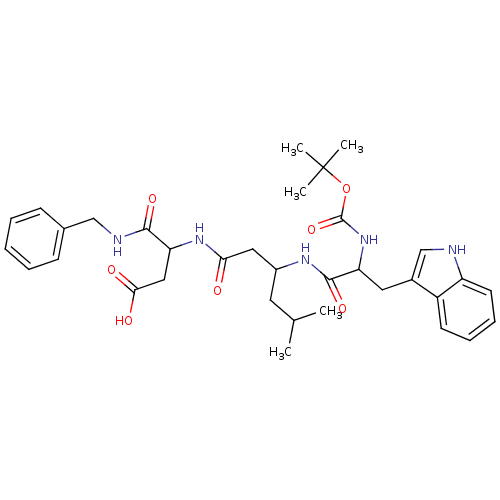 (CHEMBL356844 | N-Benzyl-3-{3-[2-tert-butoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50024332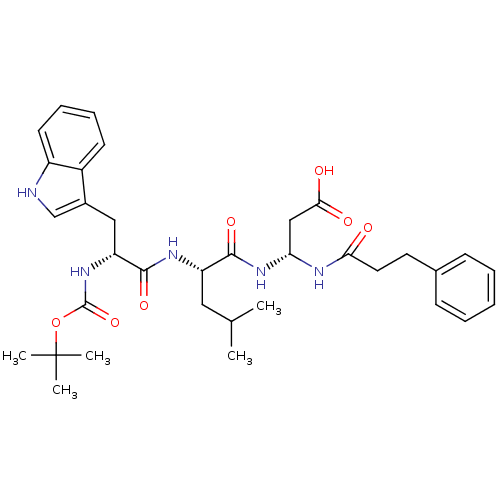 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity for binding of [125I]-(Nle11)-HG-13 to gastrin receptor on isolated rabbit gastric mucosal cells. | J Med Chem 30: 758-63 (1987) BindingDB Entry DOI: 10.7270/Q2CV4GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020567 (CHEMBL355927 | N-Benzyl-3-{3-[2-tert-butoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020572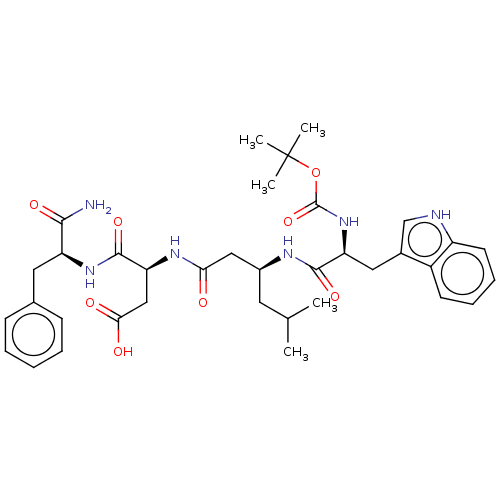 (3-{3-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020566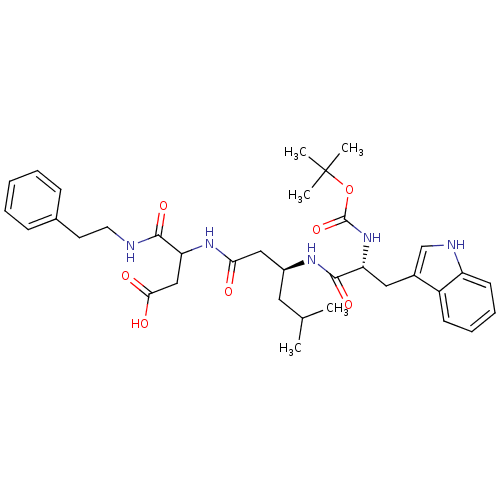 (3-{3-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020571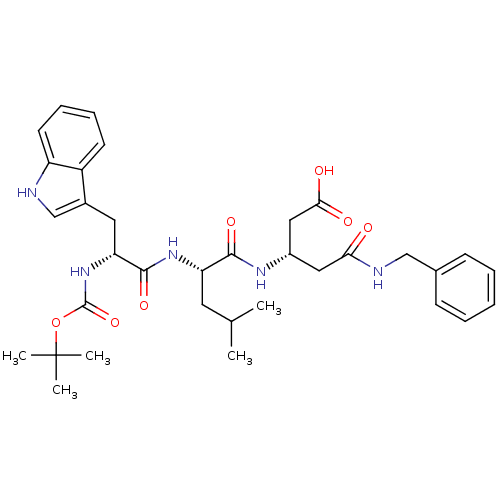 (4-Benzylcarbamoyl-3-{2-[2-tert-butoxycarbonylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50026689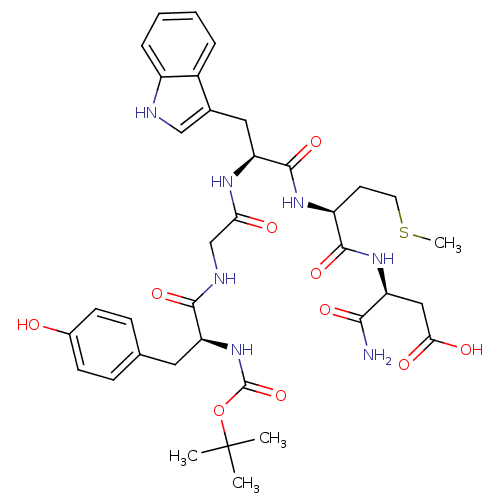 (3-{2-[2-{2-[2-tert-Butoxycarbonylamino-3-(4-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of the binding of [125I]-(Nle11)-HG-13 to Histamine H2 receptor | J Med Chem 27: 1597-601 (1985) BindingDB Entry DOI: 10.7270/Q29P30PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50024335 (3-(3-Benzyl-ureido)-N-{1-[2-tert-butoxycarbonylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity for binding of [125I]-(Nle11)-HG-13 to gastrin receptor on isolated rabbit gastric mucosal cells. | J Med Chem 30: 758-63 (1987) BindingDB Entry DOI: 10.7270/Q2CV4GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026279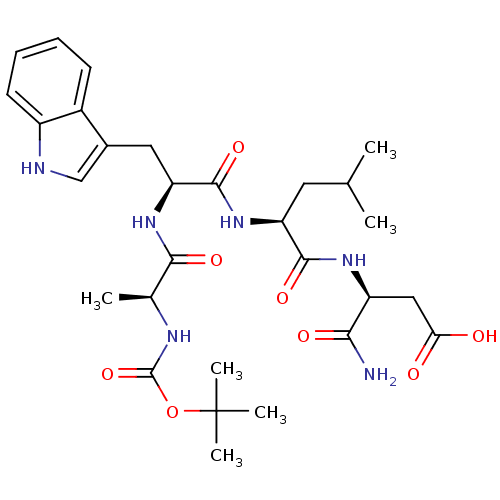 (3-{2-[2-(2-tert-Butoxycarbonylamino-propionylamino...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026297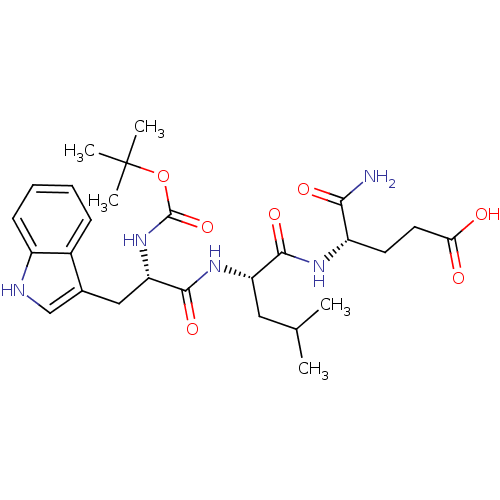 (4-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020563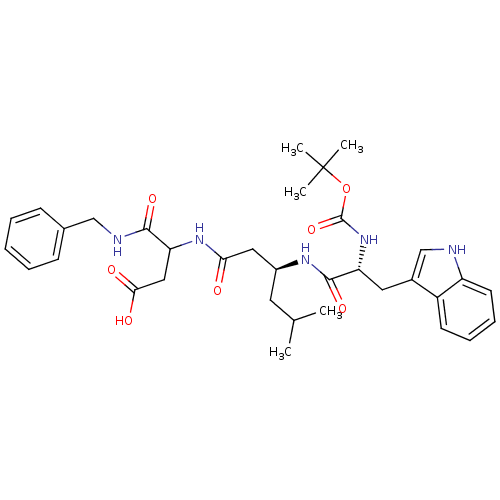 (CHEMBL151619 | N-Benzyl-3-{3-[2-tert-butoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026288 (3-{2-[2-(2-tert-Butoxycarbonylamino-propionylamino...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50452439 (CHEMBL2373213) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity for binding of [125I]-(Nle11)-HG-13 to gastrin receptor on isolated rabbit gastric mucosal cells. | J Med Chem 30: 758-63 (1987) BindingDB Entry DOI: 10.7270/Q2CV4GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020562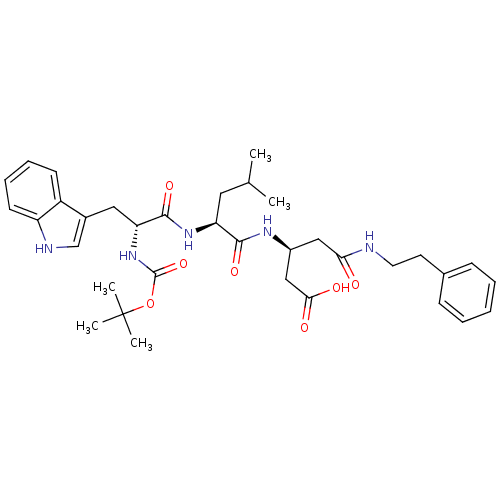 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled gCholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026298 (3-{2-[2-(2-tert-Butoxycarbonylamino-acetylamino)-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026299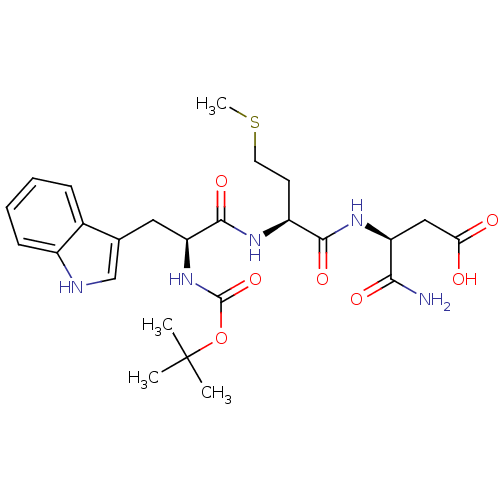 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026283 (1-(2-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026284 (2-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50026298 (3-{2-[2-(2-tert-Butoxycarbonylamino-acetylamino)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of the binding of [125I]-(Nle11)-HG-13 to Histamine H2 receptor | J Med Chem 27: 1597-601 (1985) BindingDB Entry DOI: 10.7270/Q29P30PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026282 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026286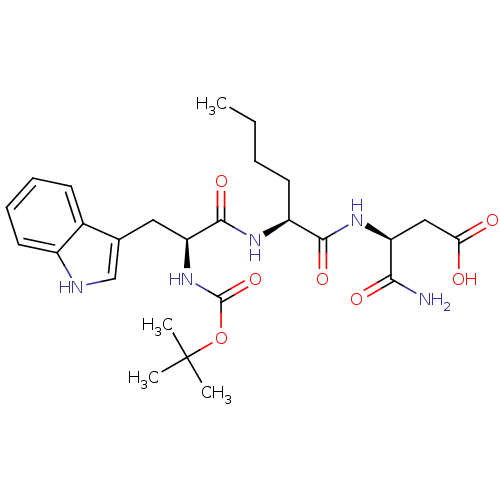 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020576 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026291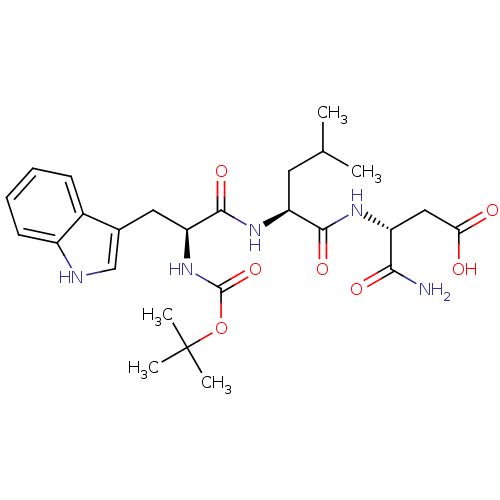 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026295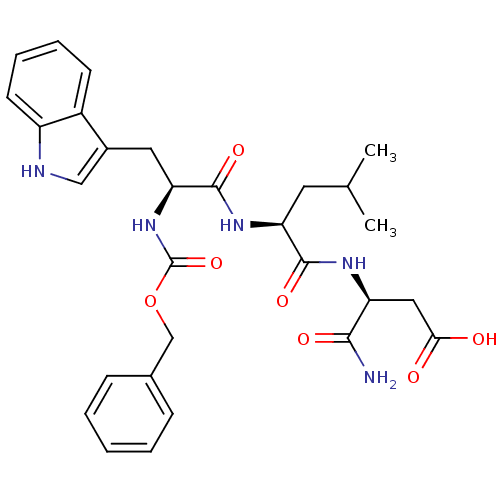 (3-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3-yl)-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50026299 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of the binding of [125I]-(Nle11)-HG-13 to Histamine H2 receptor | J Med Chem 27: 1597-601 (1985) BindingDB Entry DOI: 10.7270/Q29P30PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020577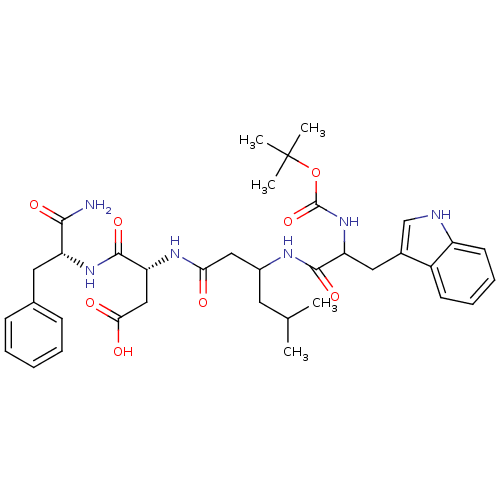 (3-{3-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020570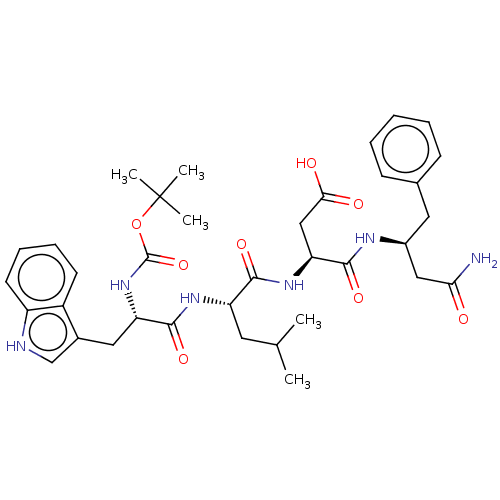 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026296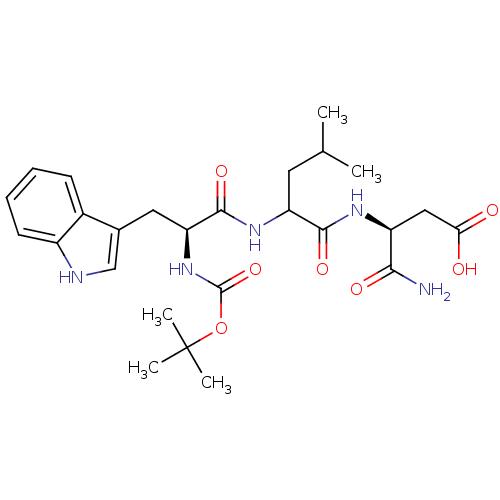 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50367238 (CHEMBL1790763) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026292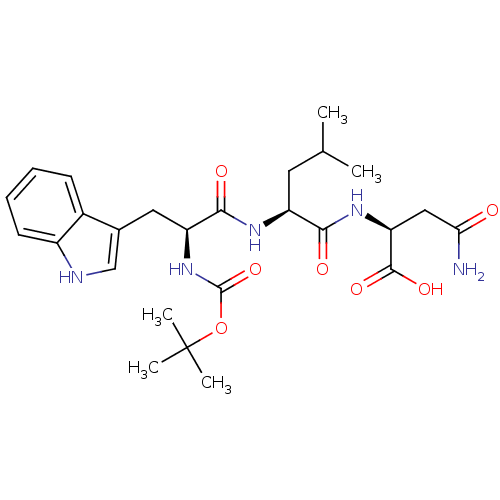 (2-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026280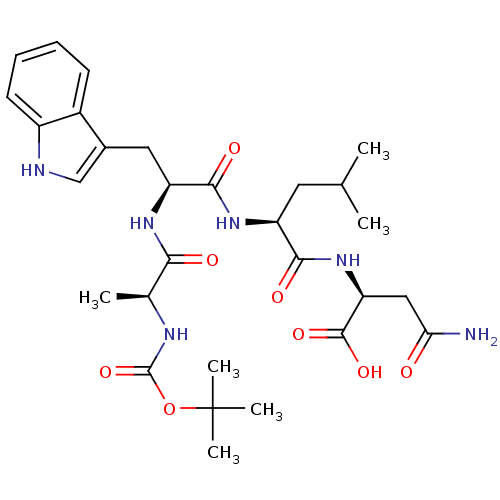 (2-{2-[2-(2-tert-Butoxycarbonylamino-propionylamino...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026290 (3-{2-[2-(2-tert-Butoxycarbonylamino-propionylamino...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50024331 (3-Benzyloxycarbonylamino-N-{1-[1-(2-butoxycarbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity for binding of [125I]-(Nle11)-HG-13 to gastrin receptor on isolated rabbit gastric mucosal cells. | J Med Chem 30: 758-63 (1987) BindingDB Entry DOI: 10.7270/Q2CV4GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50024329 (3-(3-Benzyl-ureido)-N-{1-[1-(2-butoxycarbonyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity for binding of [125I]-(Nle11)-HG-13 to gastrin receptor on isolated rabbit gastric mucosal cells. | J Med Chem 30: 758-63 (1987) BindingDB Entry DOI: 10.7270/Q2CV4GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50024333 (CHEMBL351531 | N-{1-[1-(2-Butoxycarbonyl-ethylcarb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity for binding of [125I]-(Nle11)-HG-13 to gastrin receptor on isolated rabbit gastric mucosal cells. | J Med Chem 30: 758-63 (1987) BindingDB Entry DOI: 10.7270/Q2CV4GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (RAT) | BDBM50026281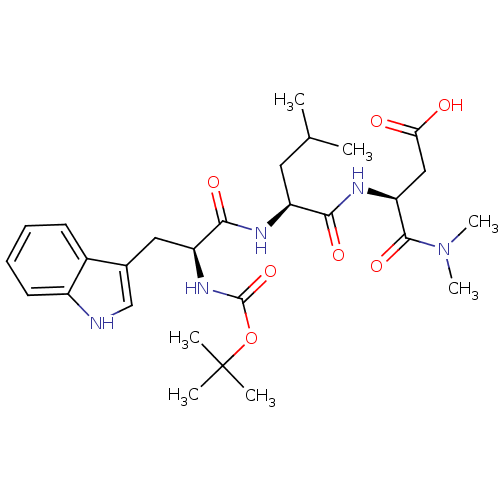 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [125I](Nle11)-HG-13 to Histamine H2 receptor in vitro | J Med Chem 28: 273-8 (1985) BindingDB Entry DOI: 10.7270/Q2ST7QC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020568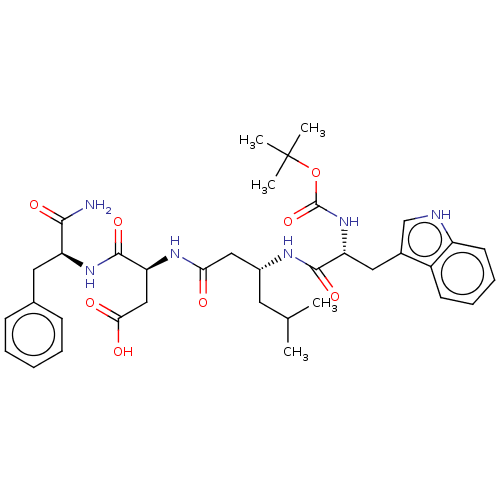 (3-{3-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Invitro inhibition of binding of [125I]-(Nle)-HG-13 labeled Cholecystokinin type B receptor on isolated gastric mucosal cells of rabbit | J Med Chem 32: 522-8 (1989) BindingDB Entry DOI: 10.7270/Q2MW2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |