

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536287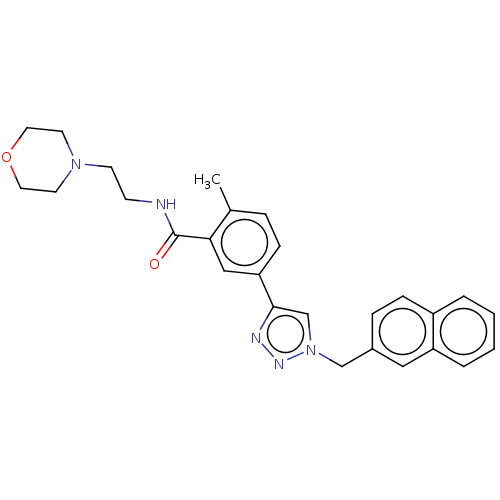 (CHEMBL4581664) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536290 (CHEMBL4525497) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536298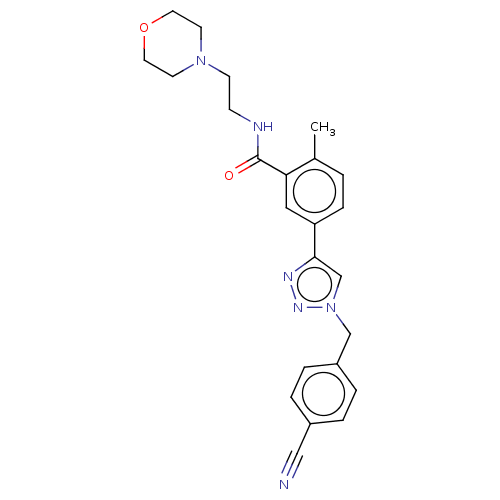 (CHEMBL4551175) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536299 (CHEMBL4525580) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536288 (CHEMBL4561751) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536294 (CHEMBL4581887) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536296 (CHEMBL4578074) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536291 (CHEMBL4513605) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536306 (CHEMBL4513836) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536293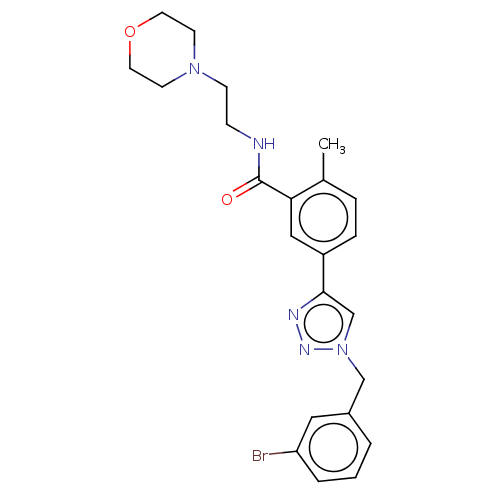 (CHEMBL4524162) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536295 (CHEMBL4516485) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536292 (CHEMBL4536296) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536285 (CHEMBL4521556) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536286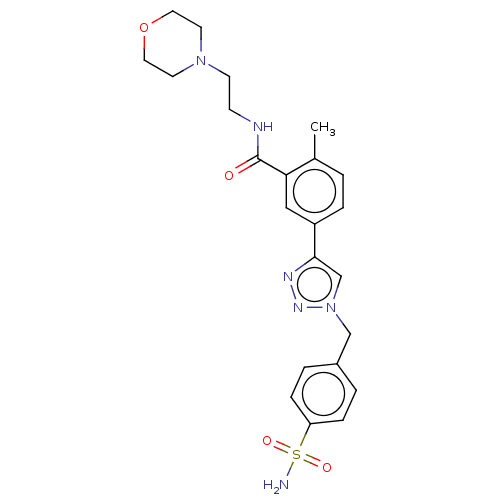 (CHEMBL4522818) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536307 (CHEMBL4541730) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536309 (CHEMBL4576458) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536303 (CHEMBL4531267) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536305 (CHEMBL4584351) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536307 (CHEMBL4541730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536292 (CHEMBL4536296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536304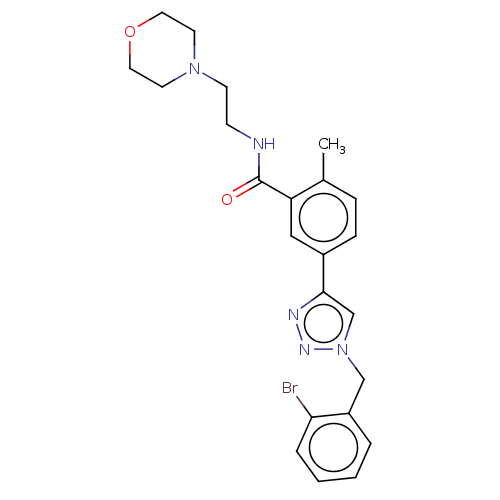 (CHEMBL4530986) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536303 (CHEMBL4531267) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536282 (CHEMBL4572737) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536289 (CHEMBL4539017) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536308 (CHEMBL4571408) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536293 (CHEMBL4524162) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536300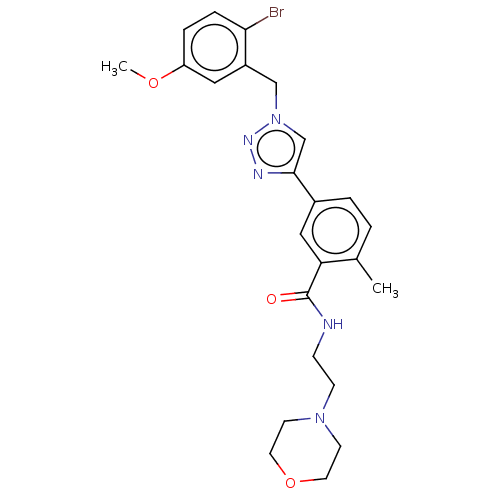 (CHEMBL4545234) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536296 (CHEMBL4578074) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536283 (CHEMBL4589486) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536305 (CHEMBL4584351) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536302 (CHEMBL4534953) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536286 (CHEMBL4522818) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536294 (CHEMBL4581887) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536297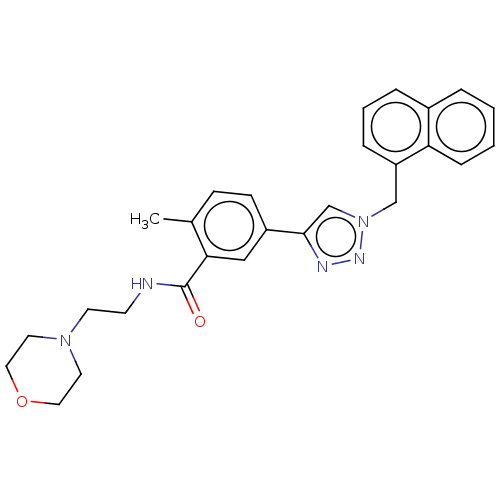 (CHEMBL4549496) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536308 (CHEMBL4571408) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536300 (CHEMBL4545234) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536301 (CHEMBL4547644) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536284 (CHEMBL4515828) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536285 (CHEMBL4521556) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536288 (CHEMBL4561751) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536295 (CHEMBL4516485) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536282 (CHEMBL4572737) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536301 (CHEMBL4547644) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536289 (CHEMBL4539017) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536302 (CHEMBL4534953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536290 (CHEMBL4525497) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536309 (CHEMBL4576458) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536297 (CHEMBL4549496) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |