 Found 619 hits with Last Name = 'seal' and Initial = 'jt'
Found 619 hits with Last Name = 'seal' and Initial = 'jt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475465
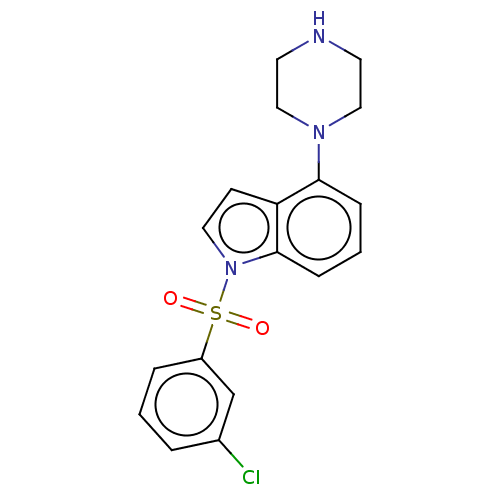
(CHEMBL196410)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-3-1-4-15(13-14)25(23,24)22-10-7-16-17(5-2-6-18(16)22)21-11-8-20-9-12-21/h1-7,10,13,20H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475462
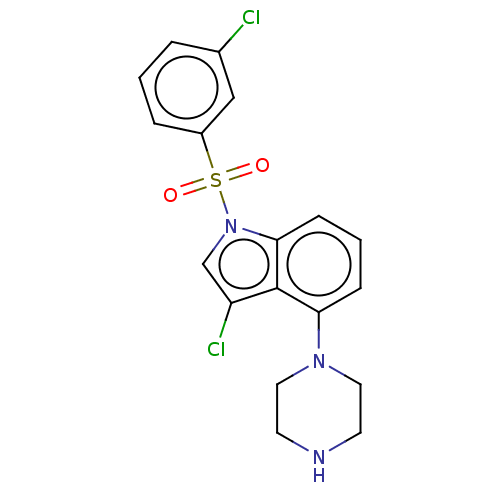
(CHEMBL371375)Show SMILES Clc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-3-1-4-14(11-13)26(24,25)23-12-15(20)18-16(5-2-6-17(18)23)22-9-7-21-8-10-22/h1-6,11-12,21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475480
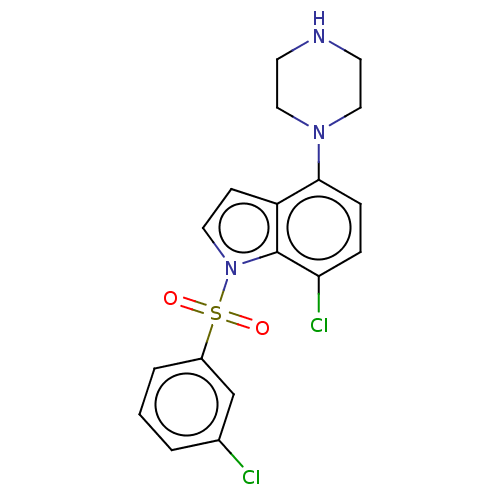
(CHEMBL193629)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(ccc(Cl)c12)N1CCNCC1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(5-4-16(20)18(15)23)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475467

(CHEMBL425015)Show SMILES Clc1cccc(c1)S(=O)(=O)c1c[nH]c2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-13-3-1-4-14(11-13)25(23,24)17-12-21-18-15(17)5-2-6-16(18)22-9-7-20-8-10-22/h1-6,11-12,20-21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475477
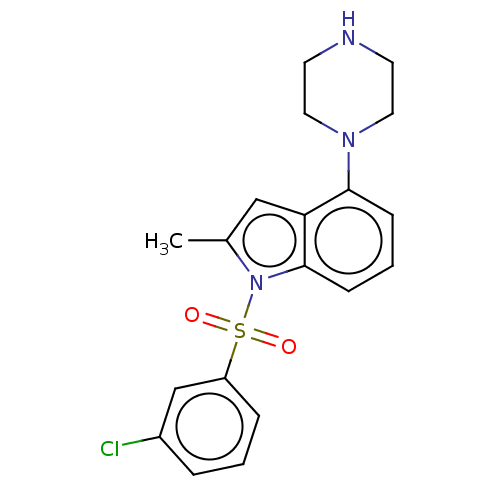
(CHEMBL372929)Show SMILES Cc1cc2c(cccc2n1S(=O)(=O)c1cccc(Cl)c1)N1CCNCC1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-12-17-18(22-10-8-21-9-11-22)6-3-7-19(17)23(14)26(24,25)16-5-2-4-15(20)13-16/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475475
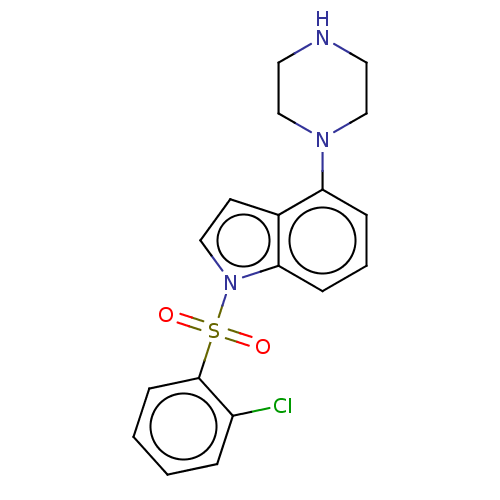
(CHEMBL372513)Show InChI InChI=1S/C18H18ClN3O2S/c19-15-4-1-2-7-18(15)25(23,24)22-11-8-14-16(5-3-6-17(14)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044607
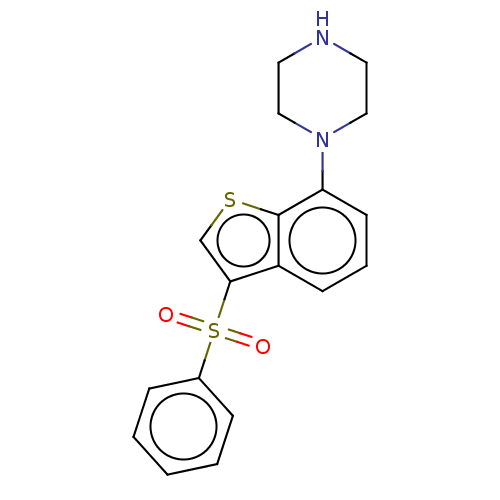
(CHEMBL372537)Show InChI InChI=1S/C18H18N2O2S2/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475463
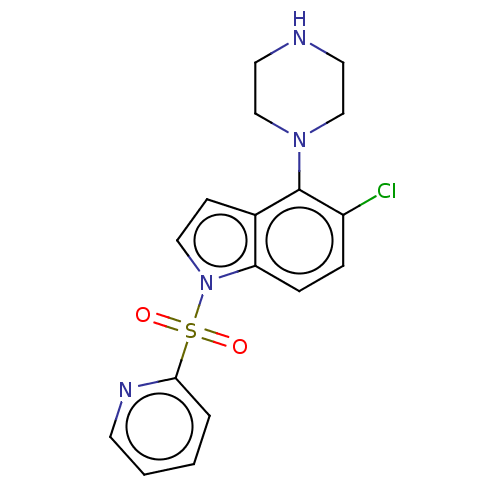
(CHEMBL194915)Show InChI InChI=1S/C17H17ClN4O2S/c18-14-4-5-15-13(17(14)21-11-8-19-9-12-21)6-10-22(15)25(23,24)16-3-1-2-7-20-16/h1-7,10,19H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475473
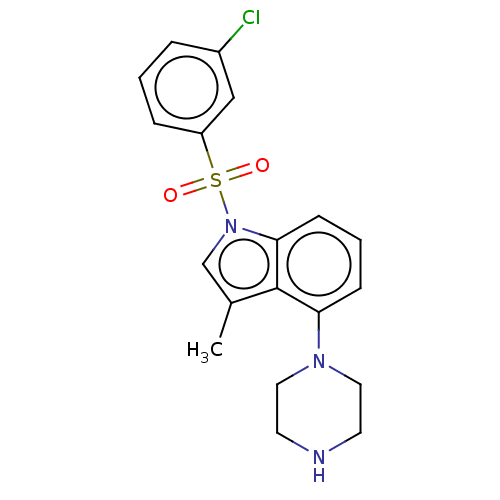
(CHEMBL194039)Show SMILES Cc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-13-23(26(24,25)16-5-2-4-15(20)12-16)18-7-3-6-17(19(14)18)22-10-8-21-9-11-22/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475479
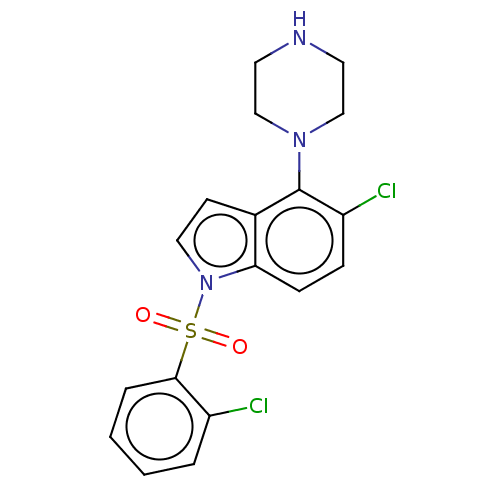
(CHEMBL371176)Show SMILES Clc1ccccc1S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-14-3-1-2-4-17(14)26(24,25)23-10-7-13-16(23)6-5-15(20)18(13)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475470

(CHEMBL370209)Show InChI InChI=1S/C17H18N4O2S/c22-24(23,17-6-1-2-8-19-17)21-11-7-14-15(4-3-5-16(14)21)20-12-9-18-10-13-20/h1-8,11,18H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475464
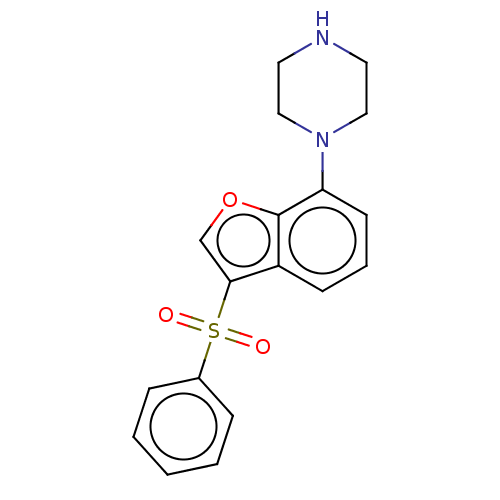
(CHEMBL197574)Show InChI InChI=1S/C18H18N2O3S/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
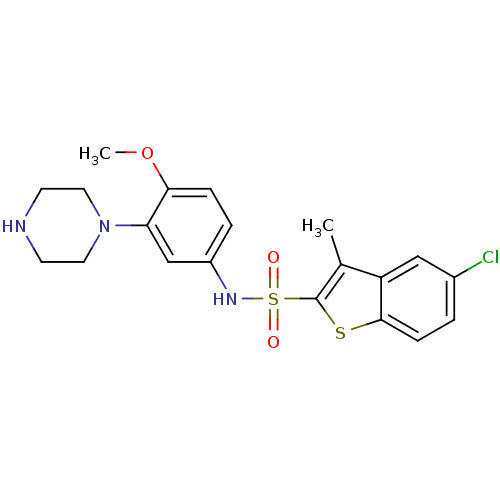
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475466
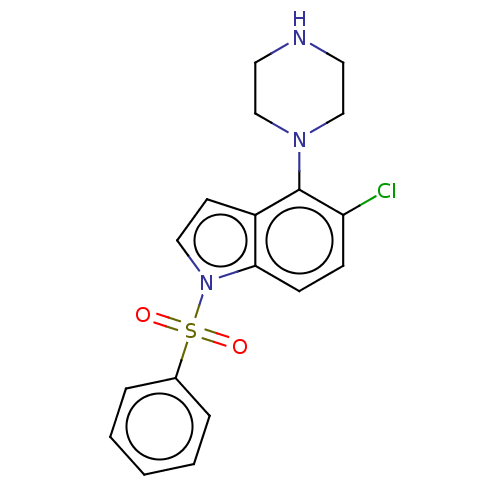
(CHEMBL193665)Show InChI InChI=1S/C18H18ClN3O2S/c19-16-6-7-17-15(18(16)21-12-9-20-10-13-21)8-11-22(17)25(23,24)14-4-2-1-3-5-14/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475471
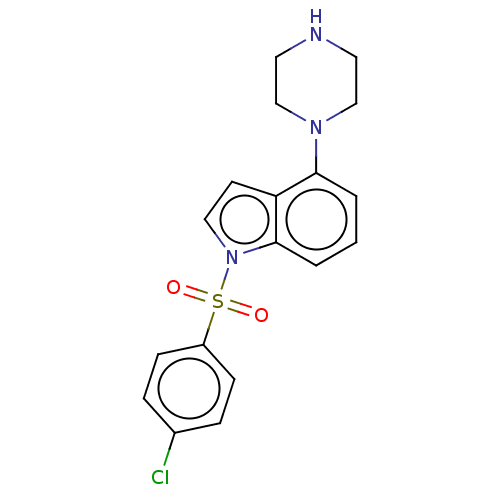
(CHEMBL371876)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-4-6-15(7-5-14)25(23,24)22-11-8-16-17(2-1-3-18(16)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475481

(CHEMBL197297)Show SMILES Cn1cc(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-22-13-18(26(24,25)15-5-2-4-14(20)12-15)16-6-3-7-17(19(16)22)23-10-8-21-9-11-23/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475478
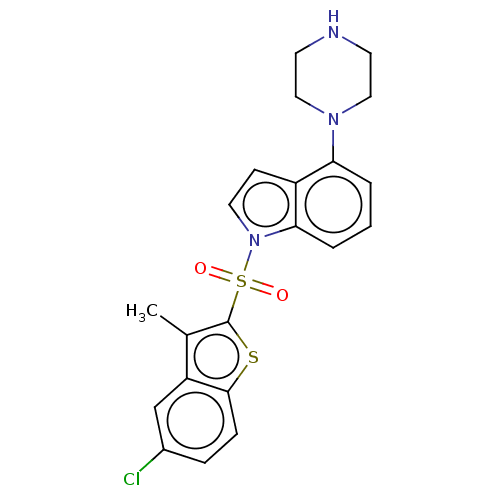
(CHEMBL196644)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2S2/c1-14-17-13-15(22)5-6-20(17)28-21(14)29(26,27)25-10-7-16-18(3-2-4-19(16)25)24-11-8-23-9-12-24/h2-7,10,13,23H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475482

(CHEMBL193379)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(ccc12)C#N Show InChI InChI=1S/C19H17ClN4O2S/c20-15-2-1-3-16(12-15)27(25,26)24-9-6-17-18(24)5-4-14(13-21)19(17)23-10-7-22-8-11-23/h1-6,9,12,22H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475472

(CHEMBL372287)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-1-3-14(4-2-13)26(24,25)23-10-7-15-17(23)6-5-16(20)18(15)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475474
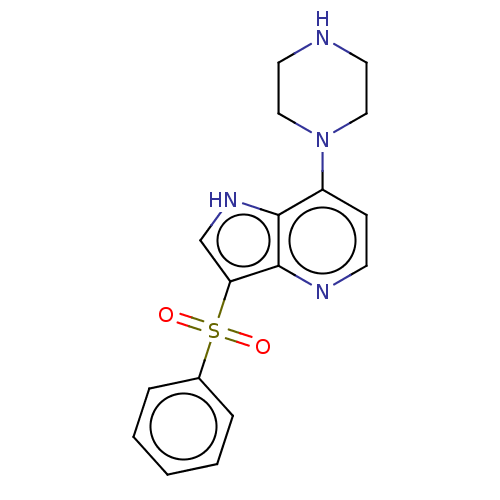
(CHEMBL426640)Show SMILES O=S(=O)(c1c[nH]c2c(ccnc12)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C17H18N4O2S/c22-24(23,13-4-2-1-3-5-13)15-12-20-16-14(6-7-19-17(15)16)21-10-8-18-9-11-21/h1-7,12,18,20H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 6 receptor of human caudate |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475476
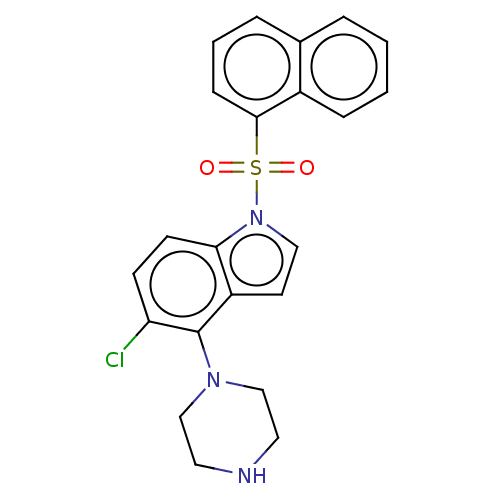
(CHEMBL196103)Show SMILES Clc1ccc2n(ccc2c1N1CCNCC1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H20ClN3O2S/c23-19-8-9-20-18(22(19)25-14-11-24-12-15-25)10-13-26(20)29(27,28)21-7-3-5-16-4-1-2-6-17(16)21/h1-10,13,24H,11-12,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 6 receptor of rat striatum |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475469

(CHEMBL196524)Show SMILES Clc1ccc2n(ccc2c1N1CCNCC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H20ClN3O2S/c23-20-7-8-21-19(22(20)25-13-10-24-11-14-25)9-12-26(21)29(27,28)18-6-5-16-3-1-2-4-17(16)15-18/h1-9,12,15,24H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475468
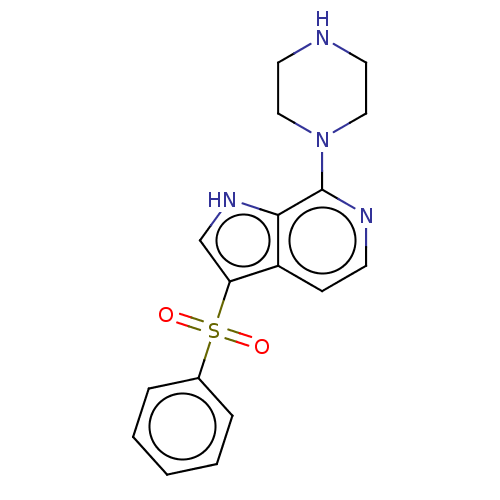
(CHEMBL366248)Show SMILES O=S(=O)(c1c[nH]c2c(nccc12)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C17H18N4O2S/c22-24(23,13-4-2-1-3-5-13)15-12-20-16-14(15)6-7-19-17(16)21-10-8-18-9-11-21/h1-7,12,18,20H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50263384
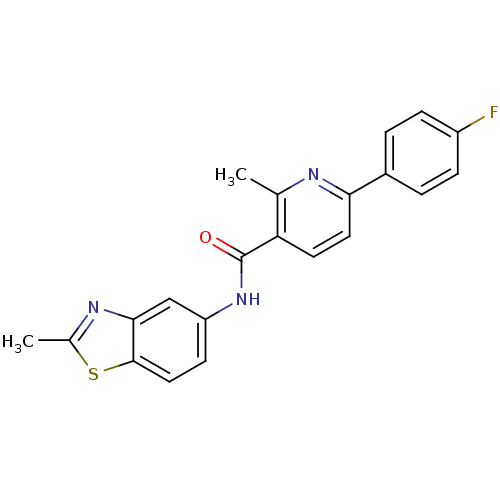
(6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazo...)Show SMILES Cc1nc2cc(NC(=O)c3ccc(nc3C)-c3ccc(F)cc3)ccc2s1 Show InChI InChI=1S/C21H16FN3OS/c1-12-17(8-9-18(23-12)14-3-5-15(22)6-4-14)21(26)25-16-7-10-20-19(11-16)24-13(2)27-20/h3-11H,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 5.3 | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5.3 acid-induced calcium influx by whole cell patch clamp a... |
Bioorg Med Chem Lett 18: 5609-13 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.105
BindingDB Entry DOI: 10.7270/Q2PZ58PN |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50566697
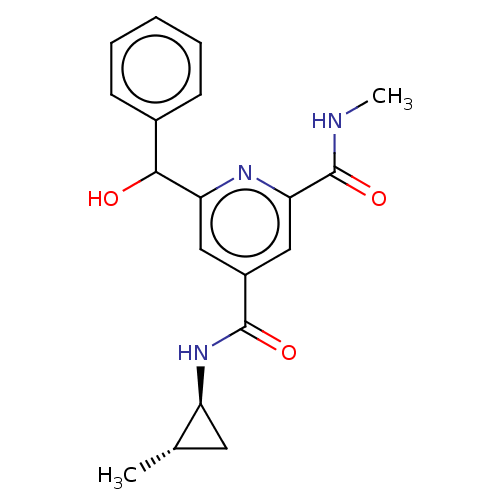
(CHEMBL4862372)Show SMILES CNC(=O)c1cc(cc(n1)C(O)c1ccccc1)C(=O)N[C@H]1C[C@@H]1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD3 BD2/BD1 Y73A mutant (1 to 435 residues) (unknown origin) measured after 30 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566686
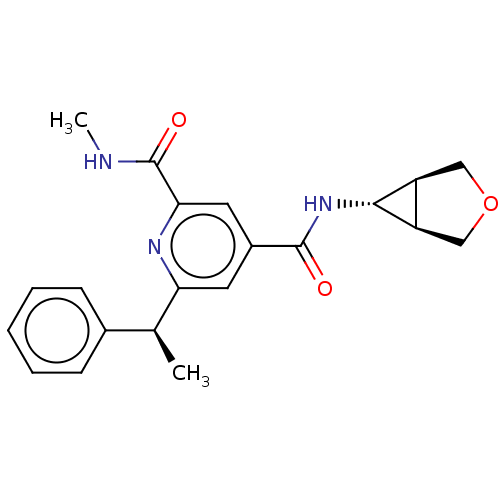
(CHEMBL4873182)Show SMILES [H][C@@]12COC[C@]1([H])[C@H]2NC(=O)c1cc(nc(c1)C(=O)NC)[C@@H](C)c1ccccc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566679
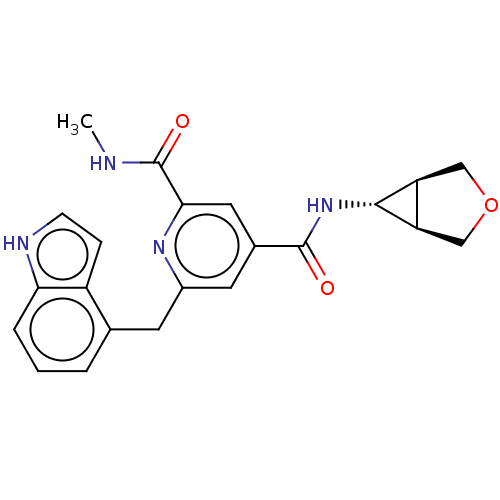
(CHEMBL4846199)Show SMILES [H][C@@]12COC[C@]1([H])[C@H]2NC(=O)c1cc(Cc2cccc3[nH]ccc23)nc(c1)C(=O)NC |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566697

(CHEMBL4862372)Show SMILES CNC(=O)c1cc(cc(n1)C(O)c1ccccc1)C(=O)N[C@H]1C[C@@H]1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50544308
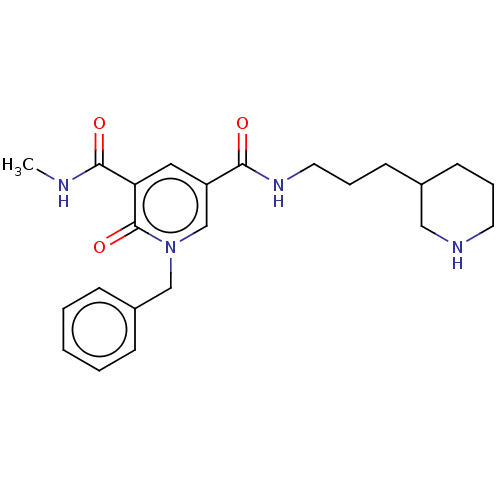
(CHEMBL4646820)Show SMILES Cl.CNC(=O)c1cc(cn(Cc2ccccc2)c1=O)C(=O)NCCCC1CCCNC1 Show InChI InChI=1S/C23H30N4O3.ClH/c1-24-22(29)20-13-19(16-27(23(20)30)15-18-7-3-2-4-8-18)21(28)26-12-6-10-17-9-5-11-25-14-17;/h2-4,7-8,13,16-17,25H,5-6,9-12,14-15H2,1H3,(H,24,29)(H,26,28);1H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-Thr-BRD4 (1 to 477 residues) BD2 domain Y390A mutant (unknown origin) incubated for 30 mins by TR-FRET assay |
J Med Chem 63: 9093-9126 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00796
BindingDB Entry DOI: 10.7270/Q27W6GS4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50544335

(CHEMBL4648431)Show SMILES CNC(=O)c1cc(cn(Cc2cccc3[nH]ccc23)c1=O)C(=O)NC1CC1 Show InChI InChI=1S/C20H20N4O3/c1-21-19(26)16-9-13(18(25)23-14-5-6-14)11-24(20(16)27)10-12-3-2-4-17-15(12)7-8-22-17/h2-4,7-9,11,14,22H,5-6,10H2,1H3,(H,21,26)(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-Thr-BRD4 (1 to 477 residues) BD2 domain Y390A mutant (unknown origin) incubated for 30 mins by TR-FRET assay |
J Med Chem 63: 9093-9126 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00796
BindingDB Entry DOI: 10.7270/Q27W6GS4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566685

(CHEMBL4860220)Show SMILES CNC(=O)c1cc(cc(n1)[C@@H](C)c1ccccc1)C(=O)NC1CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50544335

(CHEMBL4648431)Show SMILES CNC(=O)c1cc(cn(Cc2cccc3[nH]ccc23)c1=O)C(=O)NC1CC1 Show InChI InChI=1S/C20H20N4O3/c1-21-19(26)16-9-13(18(25)23-14-5-6-14)11-24(20(16)27)10-12-3-2-4-17-15(12)7-8-22-17/h2-4,7-9,11,14,22H,5-6,10H2,1H3,(H,21,26)(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566690

(CHEMBL4863255)Show SMILES [H][C@@]12COC[C@]1([H])[C@H]2NC(=O)c1cc(nc(c1)C(=O)NC)C(O)c1ccccc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566687
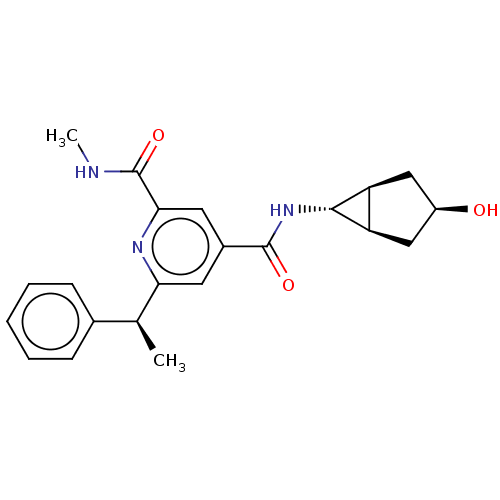
(CHEMBL4865699)Show SMILES [H][C@@]12C[C@H](O)C[C@]1([H])[C@H]2NC(=O)c1cc(nc(c1)C(=O)NC)[C@@H](C)c1ccccc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566695

(CHEMBL4846439)Show SMILES CNC(=O)c1cc(cc(n1)C(CC#N)c1ccccc1)C(=O)N[C@H]1C[C@@H]1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50544309

(CHEMBL4641088)Show SMILES CNC(=O)c1cc(cn(Cc2ccccc2)c1=O)C(=O)NCCCC1CNCCO1 Show InChI InChI=1S/C22H28N4O4/c1-23-21(28)19-12-17(15-26(22(19)29)14-16-6-3-2-4-7-16)20(27)25-9-5-8-18-13-24-10-11-30-18/h2-4,6-7,12,15,18,24H,5,8-11,13-14H2,1H3,(H,23,28)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-Thr-BRD4 (1 to 477 residues) BD2 domain Y390A mutant (unknown origin) incubated for 30 mins by TR-FRET assay |
J Med Chem 63: 9093-9126 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00796
BindingDB Entry DOI: 10.7270/Q27W6GS4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50603088
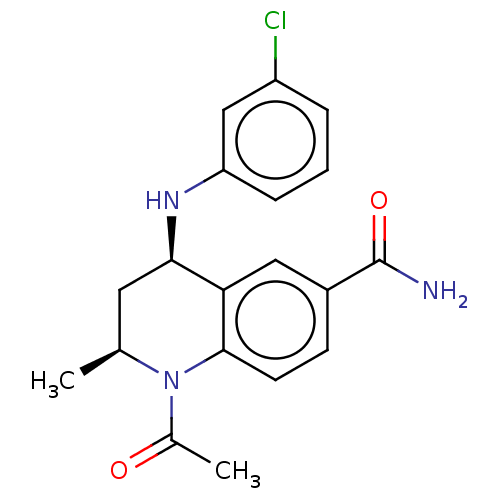
(CHEMBL5203965)Show SMILES C[C@H]1C[C@@H](Nc2cccc(Cl)c2)c2cc(ccc2N1C(C)=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01747
BindingDB Entry DOI: 10.7270/Q23B646M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566693

(CHEMBL4857098)Show SMILES [H][C@@]12C[C@@H](O)C[C@]1([H])[C@H]2NC(=O)c1cc(nc(c1)C(=O)NC)C(OC)c1ccccc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein
(Homo sapiens (Human)) | BDBM50576427

(CHEMBL4865767)Show SMILES CNC(=O)c1cc(C(=O)N[C@H]2CC[C@H](O)CC2)c(o1)[C@@H](C)c1ccccc1 |r,wU:10.9,19.21,wD:13.13,(37.7,-21.47,;37.72,-23.01,;39.06,-23.76,;40.13,-23.13,;39.09,-25.31,;40.34,-26.21,;39.89,-27.68,;40.8,-28.93,;40.3,-30.06,;42.33,-28.74,;43.08,-30.04,;44.58,-30.04,;45.33,-31.34,;44.58,-32.63,;45.33,-33.93,;43.08,-32.63,;42.33,-31.34,;38.35,-27.68,;37.84,-26.23,;37.45,-28.93,;38.07,-30.3,;35.91,-28.79,;35,-30.05,;33.47,-29.9,;32.83,-28.49,;33.73,-27.23,;35.27,-27.39,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 6His-FLAG-Tev-BRDT (1 to 397 residues) BD2 domain Y309A or Y66A mutant (unknown origin) incubated for 30 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00365
BindingDB Entry DOI: 10.7270/Q21N84ZQ |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566691
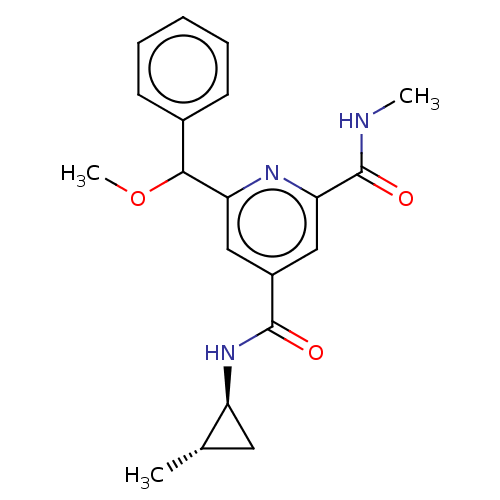
(CHEMBL4850783)Show SMILES CNC(=O)c1cc(cc(n1)C(OC)c1ccccc1)C(=O)N[C@H]1C[C@@H]1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein
(Homo sapiens (Human)) | BDBM50566697

(CHEMBL4862372)Show SMILES CNC(=O)c1cc(cc(n1)C(O)c1ccccc1)C(=O)N[C@H]1C[C@@H]1C |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRDT BD2/BD1 Y66A mutant (1 to 397 residues) (unknown origin) measured after 30 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566696
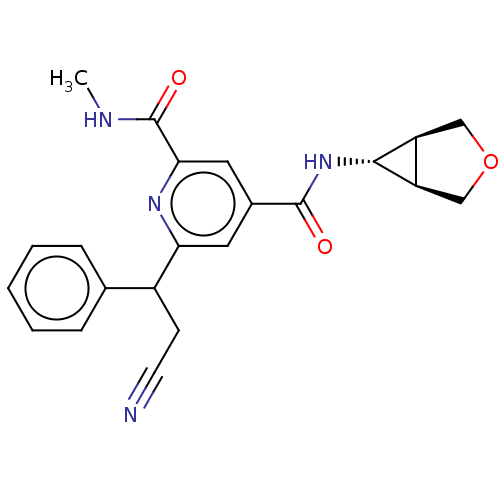
(CHEMBL4867556)Show SMILES [H][C@@]12COC[C@]1([H])[C@H]2NC(=O)c1cc(nc(c1)C(=O)NC)C(CC#N)c1ccccc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566677

(CHEMBL4863388)Show SMILES CNC(=O)c1cc(cc(Cc2ccccc2)n1)C(=O)NCCC[C@H]1OC[C@H](N)CO1 |r,wU:23.24,wD:26.28,(11.17,-12.51,;9.83,-11.74,;8.5,-12.51,;7.17,-11.74,;8.5,-14.05,;9.83,-14.83,;9.83,-16.37,;8.5,-17.13,;7.17,-16.37,;5.84,-17.14,;4.5,-16.38,;4.51,-14.84,;3.17,-14.07,;1.84,-14.84,;1.85,-16.39,;3.18,-17.15,;7.17,-14.83,;11.16,-17.14,;11.16,-18.68,;12.5,-16.38,;13.83,-17.15,;15.16,-16.38,;16.5,-17.15,;17.83,-16.38,;17.82,-14.85,;19.17,-14.08,;20.5,-14.86,;21.84,-14.1,;20.49,-16.4,;19.16,-17.16,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02155
BindingDB Entry DOI: 10.7270/Q2JH3QXP |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50575644

(CHEMBL4856853)Show SMILES CCNC(=O)c1cc(Cc2cccc3[nH]ccc23)nc(c1)-c1cn(C)nn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 6His-Thr-BRD3 (1 to 435 residues) BD2 domain Y348A or Y73A mutant (unknown origin) incubated for 30 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02156
BindingDB Entry DOI: 10.7270/Q2F193JT |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50576427

(CHEMBL4865767)Show SMILES CNC(=O)c1cc(C(=O)N[C@H]2CC[C@H](O)CC2)c(o1)[C@@H](C)c1ccccc1 |r,wU:10.9,19.21,wD:13.13,(37.7,-21.47,;37.72,-23.01,;39.06,-23.76,;40.13,-23.13,;39.09,-25.31,;40.34,-26.21,;39.89,-27.68,;40.8,-28.93,;40.3,-30.06,;42.33,-28.74,;43.08,-30.04,;44.58,-30.04,;45.33,-31.34,;44.58,-32.63,;45.33,-33.93,;43.08,-32.63,;42.33,-31.34,;38.35,-27.68,;37.84,-26.23,;37.45,-28.93,;38.07,-30.3,;35.91,-28.79,;35,-30.05,;33.47,-29.9,;32.83,-28.49,;33.73,-27.23,;35.27,-27.39,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 6His-Thr-BRD3 (1 to 435 residues) BD2 domain Y348A or Y73A mutant (unknown origin) incubated for 30 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00365
BindingDB Entry DOI: 10.7270/Q21N84ZQ |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50365262

((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...)Show SMILES Cc1nnc2[C@H](CC(=O)OC(C)(C)C)N=C(c3c(C)c(C)sc3-n12)c1ccc(Cl)cc1 |r,c:14| Show InChI InChI=1S/C23H25ClN4O2S/c1-12-13(2)31-22-19(12)20(15-7-9-16(24)10-8-15)25-17(11-18(29)30-23(4,5)6)21-27-26-14(3)28(21)22/h7-10,17H,11H2,1-6H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal BRD4 bromodomain expressed in Escherichia coli BL21(DE3) after 30 mins by luminescence proximity homogeneous assay |
J Med Chem 55: 587-96 (2012)
Article DOI: 10.1021/jm201283q
BindingDB Entry DOI: 10.7270/Q22R3SSS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data