 Found 1458 hits with Last Name = 'chow' and Initial = 'k'
Found 1458 hits with Last Name = 'chow' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tubulin beta chain
(Sus scrofa) | BDBM50485941

(CHEMBL2181004)Show SMILES COc1ccc(CN[C@H]2CCc3cc(OC)c(OC)c(OC)c3-c3ccc(OC)c(=O)cc23)cc1 |r| Show InChI InChI=1S/C28H31NO6/c1-31-19-9-6-17(7-10-19)16-29-22-12-8-18-14-25(33-3)27(34-4)28(35-5)26(18)20-11-13-24(32-2)23(30)15-21(20)22/h6-7,9-11,13-15,22,29H,8,12,16H2,1-5H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485945

(CHEMBL2181003)Show SMILES COc1cc2CC[C@H](NCc3ccc(cc3)[N+]([O-])=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28N2O7/c1-33-23-12-10-19-20(14-22(23)30)21(28-15-16-5-8-18(9-6-16)29(31)32)11-7-17-13-24(34-2)26(35-3)27(36-4)25(17)19/h5-6,8-10,12-14,21,28H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485942

(CHEMBL2181002)Show SMILES COc1cc2CC[C@H](NCc3cc(F)c(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H26F3NO5/c1-33-22-8-6-16-17(12-21(22)32)20(31-13-14-9-18(28)25(30)19(29)10-14)7-5-15-11-23(34-2)26(35-3)27(36-4)24(15)16/h6,8-12,20,31H,5,7,13H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485950
(CHEMBL2181009)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-11-9-19-20(14-22(23)30)21(29-15-16-6-5-7-18(28)12-16)10-8-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-7,9,11-14,21,29H,8,10,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485944

(CHEMBL2181006)Show SMILES COc1cc2CC[C@H](NCc3ccc(Cl)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28ClNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485943
(CHEMBL2181001)Show SMILES COc1cc2CC[C@H](NCc3cc(F)cc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-8-6-19-20(13-22(23)31)21(30-14-15-9-17(28)12-18(29)10-15)7-5-16-11-24(33-2)26(34-3)27(35-4)25(16)19/h6,8-13,21,30H,5,7,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485946
(CHEMBL2181000)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-10-7-17-18(13-22(23)31)21(30-14-15-5-8-19(28)20(29)11-15)9-6-16-12-24(33-2)26(34-3)27(35-4)25(16)17/h5,7-8,10-13,21,30H,6,9,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485947
(CHEMBL2181008)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485949

(CHEMBL2180999)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3F)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-22-11-9-17-18(13-21(22)31)20(30-14-16-6-5-7-19(28)25(16)29)10-8-15-12-23(33-2)26(34-3)27(35-4)24(15)17/h5-7,9,11-13,20,30H,8,10,14H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485948
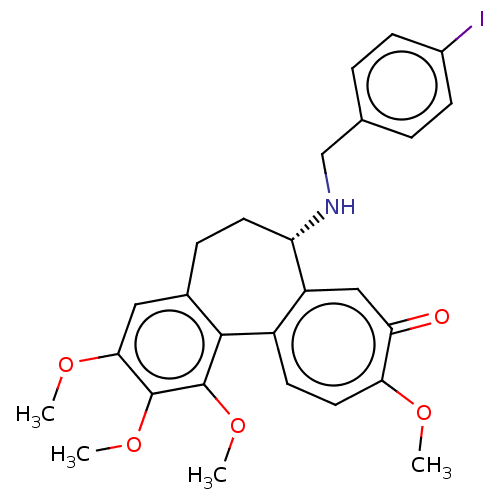
(CHEMBL2181007)Show SMILES COc1cc2CC[C@H](NCc3ccc(I)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28INO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50393371
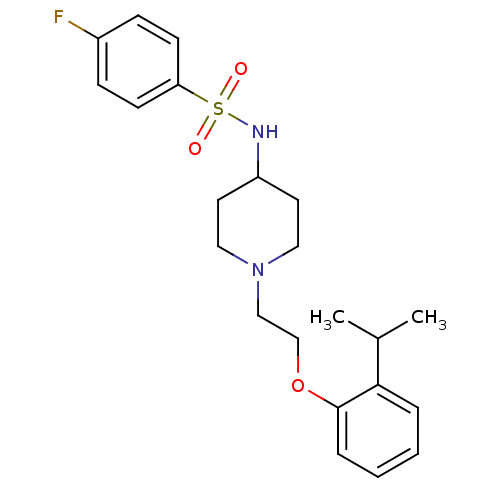
(CHEMBL2159465)Show SMILES CC(C)c1ccccc1OCCN1CCC(CC1)NS(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C22H29FN2O3S/c1-17(2)21-5-3-4-6-22(21)28-16-15-25-13-11-19(12-14-25)24-29(26,27)20-9-7-18(23)8-10-20/h3-10,17,19,24H,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7b receptor expressed in HEK293 cells after 1 hr |
Eur J Med Chem 56: 348-360 (2012)
Article DOI: 10.1016/j.ejmech.2012.07.043
BindingDB Entry DOI: 10.7270/Q28P61N6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50279520
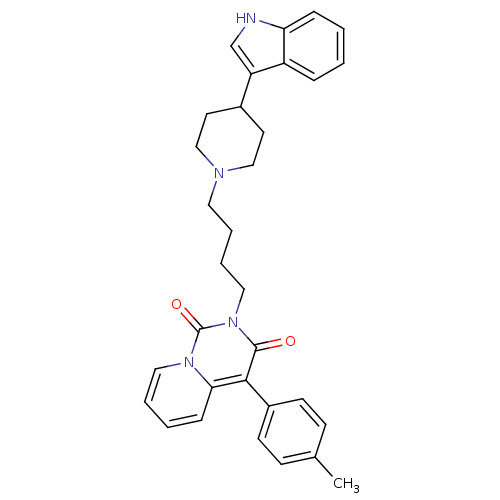
(2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-p...)Show SMILES Cc1ccc(cc1)-c1c2ccccn2c(=O)n(CCCCN2CCC(CC2)c2c[nH]c3ccccc23)c1=O Show InChI InChI=1S/C32H34N4O2/c1-23-11-13-25(14-12-23)30-29-10-4-5-18-35(29)32(38)36(31(30)37)19-7-6-17-34-20-15-24(16-21-34)27-22-33-28-9-3-2-8-26(27)28/h2-5,8-14,18,22,24,33H,6-7,15-17,19-21H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from 5HTT in rat brain membranes |
Eur J Med Chem 44: 1710-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.021
BindingDB Entry DOI: 10.7270/Q22N5249 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| DrugBank
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by microbeta plate reader method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112765
BindingDB Entry DOI: 10.7270/Q2ZC86K7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485940

(CHEMBL2181005)Show SMILES COc1cc2CC[C@H](NCc3ccc(Br)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28BrNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2BR (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Ketanserin from human 5-HT2A receptor expressed in rat cortex tissue incubated for 30 mins by liquid scintillation counting meth... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from dopamine D2 receptor in rat straitum tissue after 20 mins by scintillation counting |
Eur J Med Chem 46: 4474-88 (2011)
Article DOI: 10.1016/j.ejmech.2011.07.022
BindingDB Entry DOI: 10.7270/Q2Z0395D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM85222
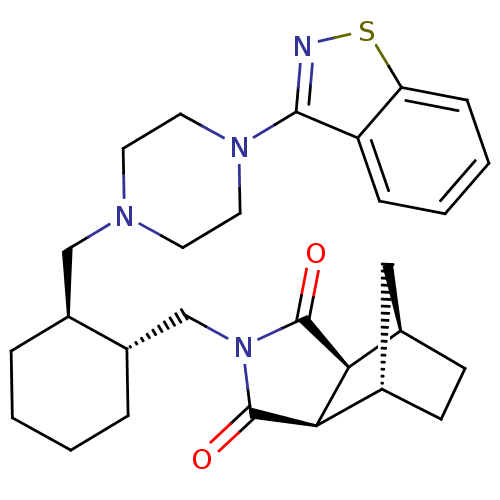
(CAS_441351-20-8 | Lurasidone | SM 13496)Show SMILES O=C1[C@H]2[C@@H]3CC[C@@H](C3)[C@H]2C(=O)N1C[C@@H]1CCCC[C@H]1CN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C28H36N4O2S/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2/t18-,19+,20-,21-,24+,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5HT7 receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50253281
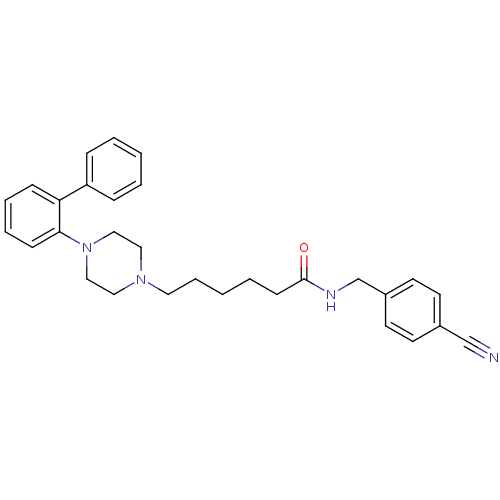
(CHEMBL522691 | N-(4-Cyanophenylmethyl)-4-(2-diphen...)Show SMILES O=C(CCCCCN1CCN(CC1)c1ccccc1-c1ccccc1)NCc1ccc(cc1)C#N Show InChI InChI=1S/C30H34N4O/c31-23-25-14-16-26(17-15-25)24-32-30(35)13-5-2-8-18-33-19-21-34(22-20-33)29-12-7-6-11-28(29)27-9-3-1-4-10-27/h1,3-4,6-7,9-12,14-17H,2,5,8,13,18-22,24H2,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7b receptor expressed in HEK293 cells after 1 hr |
Eur J Med Chem 56: 348-360 (2012)
Article DOI: 10.1016/j.ejmech.2012.07.043
BindingDB Entry DOI: 10.7270/Q28P61N6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50279541

(2-(4-(4-(1H-indol-3-yl)piperidin-1-yl)butyl)-4-(2-...)Show SMILES Fc1ccccc1-c1c2ccccn2c(=O)n(CCCCN2CCC(CC2)c2c[nH]c3ccccc23)c1=O |(-3.9,-32.75,;-5.23,-31.98,;-5.23,-30.44,;-6.57,-29.67,;-7.91,-30.46,;-7.9,-31.99,;-6.57,-32.75,;-6.56,-34.29,;-7.89,-35.07,;-9.23,-34.29,;-10.57,-35.07,;-10.57,-36.62,;-9.23,-37.39,;-7.89,-36.62,;-6.55,-37.39,;-6.55,-38.93,;-5.21,-36.61,;-3.88,-37.38,;-2.54,-36.61,;-1.21,-37.38,;.12,-36.61,;1.46,-37.38,;1.45,-38.93,;2.78,-39.7,;4.11,-38.94,;4.12,-37.39,;2.78,-36.61,;5.45,-39.71,;5.61,-41.24,;7.11,-41.56,;7.88,-40.23,;9.39,-39.92,;9.87,-38.46,;8.83,-37.31,;7.33,-37.63,;6.86,-39.08,;-5.21,-35.06,;-3.88,-34.29,)| Show InChI InChI=1S/C31H31FN4O2/c32-26-11-3-1-10-24(26)29-28-13-5-6-17-35(28)31(38)36(30(29)37)18-8-7-16-34-19-14-22(15-20-34)25-21-33-27-12-4-2-9-23(25)27/h1-6,9-13,17,21-22,33H,7-8,14-16,18-20H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from 5HTT in rat brain membranes |
Eur J Med Chem 44: 1710-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.021
BindingDB Entry DOI: 10.7270/Q22N5249 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50419052

(SB-399885)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)Nc1cc(Cl)cc(Cl)c1OC Show InChI InChI=1S/C18H21Cl2N3O4S/c1-26-17-4-3-13(11-16(17)23-7-5-21-6-8-23)28(24,25)22-15-10-12(19)9-14(20)18(15)27-2/h3-4,9-11,21-22H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00224
BindingDB Entry DOI: 10.7270/Q2X3528B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM30708
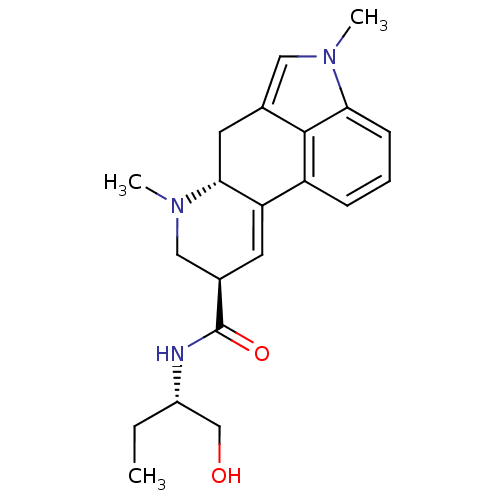
((6aR,9R)-4,7-dimethyl-N-[(1S)-1-methylolpropyl]-6,...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2CR (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in HEK293 after 1 hr |
Eur J Med Chem 60: 42-50 (2013)
Article DOI: 10.1016/j.ejmech.2012.11.042
BindingDB Entry DOI: 10.7270/Q21V5G98 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50579346

(CHEMBL4867565)Show SMILES Fc1cccc(c1)S(=O)(=O)n1ccc2c(nc3ccccc3c12)N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00224
BindingDB Entry DOI: 10.7270/Q2X3528B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7b receptor expressed in HEK293 cells after 1 hr |
Eur J Med Chem 56: 348-360 (2012)
Article DOI: 10.1016/j.ejmech.2012.07.043
BindingDB Entry DOI: 10.7270/Q28P61N6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM10989
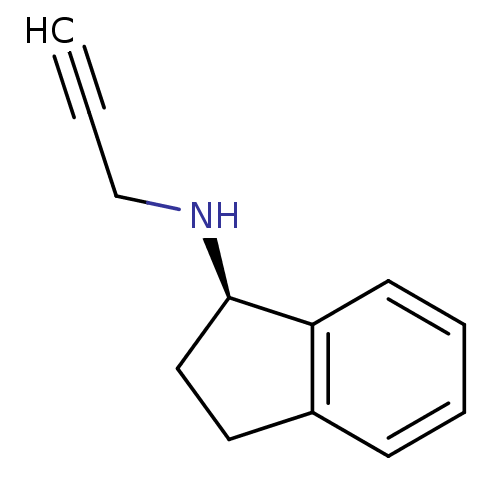
((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...)Show InChI InChI=1S/C12H13N/c1-2-9-13-12-8-7-10-5-3-4-6-11(10)12/h1,3-6,12-13H,7-9H2/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by microbeta plate reader method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112765
BindingDB Entry DOI: 10.7270/Q2ZC86K7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00224
BindingDB Entry DOI: 10.7270/Q2X3528B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT6R expressed in HEK293 cell membranes after 1 hr |
Bioorg Med Chem 26: 3588-3595 (2018)
Article DOI: 10.1016/j.bmc.2018.05.033
BindingDB Entry DOI: 10.7270/Q2BC4219 |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50014846
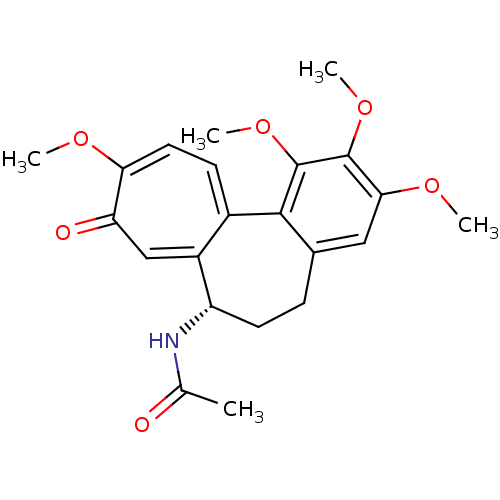
((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM85222

(CAS_441351-20-8 | Lurasidone | SM 13496)Show SMILES O=C1[C@H]2[C@@H]3CC[C@@H](C3)[C@H]2C(=O)N1C[C@@H]1CCCC[C@H]1CN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C28H36N4O2S/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2/t18-,19+,20-,21-,24+,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2 receptor expressed in HEK cells incubated for 1 hr by Cheng-Prusoff analysis based microbeta scintillat... |
Eur J Med Chem 180: 383-397 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.027
BindingDB Entry DOI: 10.7270/Q23B63H7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from recombinant human D2 receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting ... |
Eur J Med Chem 166: 144-158 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.031
BindingDB Entry DOI: 10.7270/Q2M61PPW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM105212

(2-(4-Nonyl-piperazin-1-yl)-6-nitroquinoline (9))Show SMILES CCCCCCCCCN1CCN(CC1)c1ccc2cc(ccc2n1)[N+]([O-])=O Show InChI InChI=1S/C22H32N4O2/c1-2-3-4-5-6-7-8-13-24-14-16-25(17-15-24)22-12-9-19-18-20(26(27)28)10-11-21(19)23-22/h9-12,18H,2-8,13-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.7 | 25 |
University of Troms£
| Assay Description
The assay was performed in accordance with the method described by Owens et al. with slight modifications. Rat cerebral cortex was homogenized in 30... |
Chem Biol Drug Des 81: 695-706 (2013)
Article DOI: 10.1111/cbdd.12116
BindingDB Entry DOI: 10.7270/Q2542M7W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM30708

((6aR,9R)-4,7-dimethyl-N-[(1S)-1-methylolpropyl]-6,...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2BR (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50151982

(5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...)Show SMILES NC(=O)c1cc2cc(ccc2o1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 Show InChI InChI=1S/C26H27N5O2/c27-16-18-4-6-23-22(13-18)19(17-29-23)3-1-2-8-30-9-11-31(12-10-30)21-5-7-24-20(14-21)15-25(33-24)26(28)32/h4-7,13-15,17,29H,1-3,8-12H2,(H2,28,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-imipramine from human serotonin transporter expressed in HEK293 cells membranes incubated for 30 mins by microbeta scintillation... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50393395
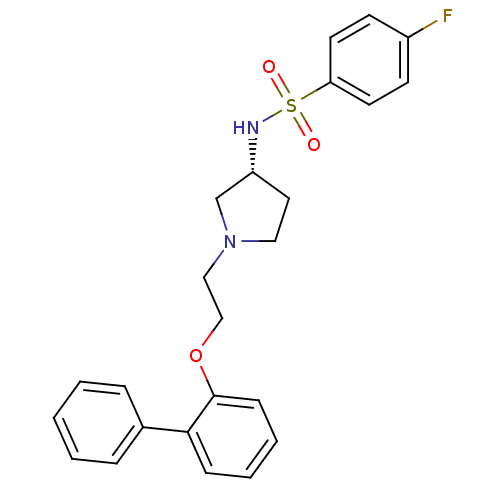
(CHEMBL2159489)Show SMILES Fc1ccc(cc1)S(=O)(=O)N[C@@H]1CCN(CCOc2ccccc2-c2ccccc2)C1 |r| Show InChI InChI=1S/C24H25FN2O3S/c25-20-10-12-22(13-11-20)31(28,29)26-21-14-15-27(18-21)16-17-30-24-9-5-4-8-23(24)19-6-2-1-3-7-19/h1-13,21,26H,14-18H2/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7b receptor expressed in HEK293 cells after 1 hr |
Eur J Med Chem 56: 348-360 (2012)
Article DOI: 10.1016/j.ejmech.2012.07.043
BindingDB Entry DOI: 10.7270/Q28P61N6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579339
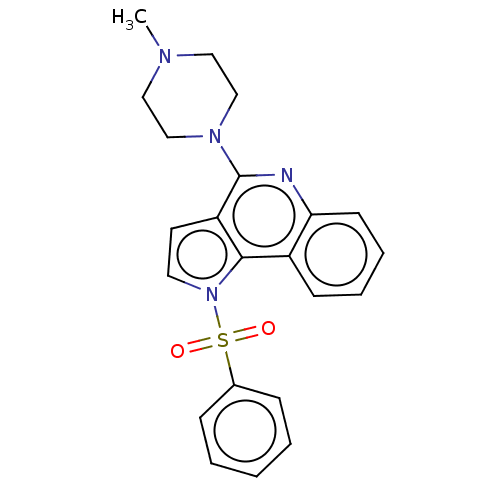
(CHEMBL4865309)Show SMILES CN1CCN(CC1)c1nc2ccccc2c2n(ccc12)S(=O)(=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00224
BindingDB Entry DOI: 10.7270/Q2X3528B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579341

(CHEMBL4860809)Show SMILES CN1CCN(CC1)c1nc2ccccc2c2n(ccc12)S(=O)(=O)c1cccc(F)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00224
BindingDB Entry DOI: 10.7270/Q2X3528B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50579342

(CHEMBL4872586)Show SMILES CN1CCN(CC1)c1nc2ccccc2c2n(ccc12)S(=O)(=O)c1cccc(Cl)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00224
BindingDB Entry DOI: 10.7270/Q2X3528B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85222

(CAS_441351-20-8 | Lurasidone | SM 13496)Show SMILES O=C1[C@H]2[C@@H]3CC[C@@H](C3)[C@H]2C(=O)N1C[C@@H]1CCCC[C@H]1CN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C28H36N4O2S/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2/t18-,19+,20-,21-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Ketanserin from human 5-HT2A receptor expressed in rat cortex tissue incubated for 30 mins by liquid scintillation counting meth... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50504436
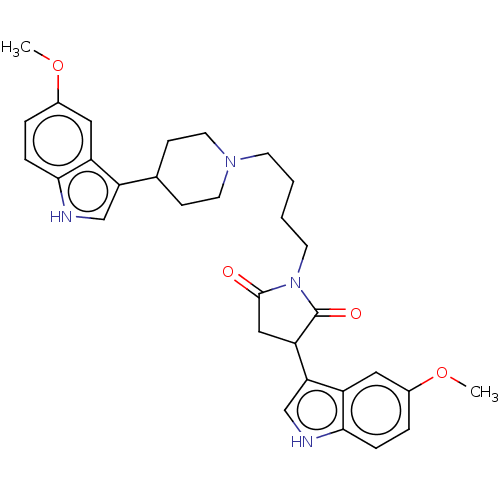
(CHEMBL4591440)Show SMILES COc1ccc2[nH]cc(C3CC(=O)N(CCCCN4CCC(CC4)c4c[nH]c5ccc(OC)cc45)C3=O)c2c1 Show InChI InChI=1S/C31H36N4O4/c1-38-21-5-7-28-23(15-21)26(18-32-28)20-9-13-34(14-10-20)11-3-4-12-35-30(36)17-25(31(35)37)27-19-33-29-8-6-22(39-2)16-24(27)29/h5-8,15-16,18-20,25,32-33H,3-4,9-14,17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50010859
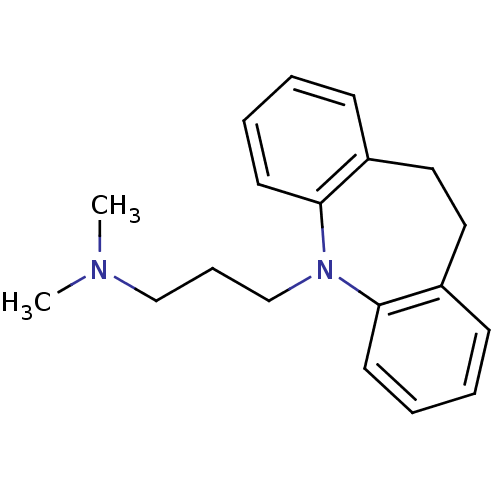
(CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...)Show InChI InChI=1S/C19H24N2/c1-20(2)14-7-15-21-18-10-5-3-8-16(18)12-13-17-9-4-6-11-19(17)21/h3-6,8-11H,7,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-imipramine from human serotonin transporter expressed in HEK293 cells membranes incubated for 30 mins by microbeta scintillation... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50354102
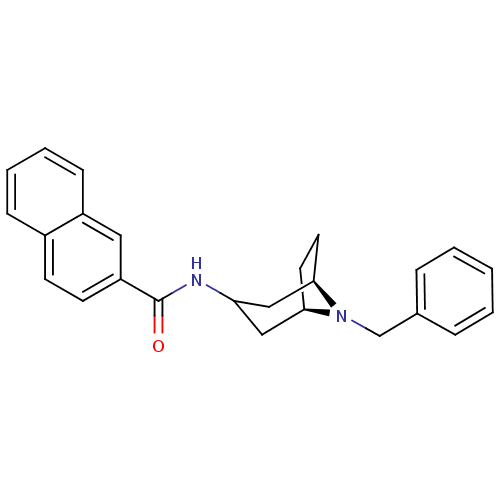
(CHEMBL1836107)Show SMILES O=C(NC1C[C@H]2CC[C@H](C1)N2Cc1ccccc1)c1ccc2ccccc2c1 |r,TLB:2:3:10:6.7| Show InChI InChI=1S/C25H26N2O/c28-25(21-11-10-19-8-4-5-9-20(19)14-21)26-22-15-23-12-13-24(16-22)27(23)17-18-6-2-1-3-7-18/h1-11,14,22-24H,12-13,15-17H2,(H,26,28)/t23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A in rat brain cerebral cortex after 20 mins by scintillation counting |
Eur J Med Chem 46: 4474-88 (2011)
Article DOI: 10.1016/j.ejmech.2011.07.022
BindingDB Entry DOI: 10.7270/Q2Z0395D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]8OH-DPAT from 5HT1A in rat brain cerebral cortex after 15 mins by scintillation counting |
Eur J Med Chem 46: 4474-88 (2011)
Article DOI: 10.1016/j.ejmech.2011.07.022
BindingDB Entry DOI: 10.7270/Q2Z0395D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data