 Found 34 hits with Last Name = 'schmitz' and Initial = 'k'
Found 34 hits with Last Name = 'schmitz' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50526216
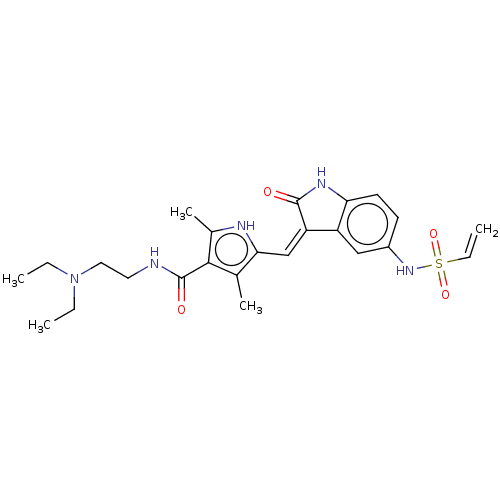
(CHEMBL4553725)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(NS(=O)(=O)C=C)cc23)c1C Show InChI InChI=1S/C24H31N5O4S/c1-6-29(7-2)12-11-25-24(31)22-15(4)21(26-16(22)5)14-19-18-13-17(28-34(32,33)8-3)9-10-20(18)27-23(19)30/h8-10,13-14,26,28H,3,6-7,11-12H2,1-2,4-5H3,(H,25,31)(H,27,30)/b19-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Irreversible inhibition of recombinant human FLT3 D835Y mutant (571 to 993 residues) expressed in baculovirus expression system preincubated for 5 to... |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50526217
(CHEMBL4442997)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(NC(=O)CCl)cc23)c1C Show InChI InChI=1S/C24H30ClN5O3/c1-5-30(6-2)10-9-26-24(33)22-14(3)20(27-15(22)4)12-18-17-11-16(28-21(31)13-25)7-8-19(17)29-23(18)32/h7-8,11-12,27H,5-6,9-10,13H2,1-4H3,(H,26,33)(H,28,31)(H,29,32)/b18-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Irreversible inhibition of recombinant human FLT3 D835Y mutant (571 to 993 residues) expressed in baculovirus expression system preincubated for 5 to... |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Androgen receptor [646-919]
(Homo sapiens (Human)) | BDBM227645
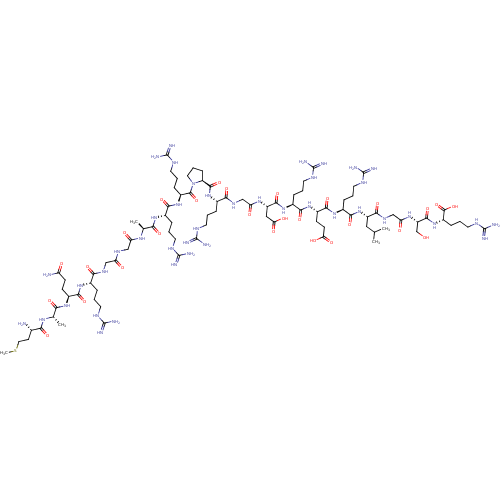
(Bag-1L (1-20) | MAQRGGARRPRGDRERLGSR)Show SMILES CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C89H160N42O27S/c1-43(2)36-56(72(147)116-41-64(137)120-58(42-132)79(154)129-55(82(157)158)20-12-33-112-89(104)105)130-75(150)51(18-10-31-110-87(100)101)124-77(152)53(23-25-65(138)139)126-73(148)50(17-9-30-109-86(98)99)125-78(153)57(37-66(140)141)119-63(136)40-115-71(146)48(15-7-28-107-84(94)95)127-80(155)59-21-13-34-131(59)81(156)54(19-11-32-111-88(102)103)128-74(149)49(16-8-29-108-85(96)97)121-67(142)44(3)117-62(135)39-113-61(134)38-114-70(145)47(14-6-27-106-83(92)93)123-76(151)52(22-24-60(91)133)122-68(143)45(4)118-69(144)46(90)26-35-159-5/h43-59,132H,6-42,90H2,1-5H3,(H2,91,133)(H,113,134)(H,114,145)(H,115,146)(H,116,147)(H,117,135)(H,118,144)(H,119,136)(H,120,137)(H,121,142)(H,122,143)(H,123,151)(H,124,152)(H,125,153)(H,126,148)(H,127,155)(H,128,149)(H,129,154)(H,130,150)(H,138,139)(H,140,141)(H,157,158)(H4,92,93,106)(H4,94,95,107)(H4,96,97,108)(H4,98,99,109)(H4,100,101,110)(H4,102,103,111)(H4,104,105,112)/t44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Karlsruhe Institute of Technology
| Assay Description
We performed a mammalian one-hybrid assay in HeLa cells, where we transfected a construct coding for a Gal4DBD-BagN128 fusion or just Gal4DBD alone t... |
J Biol Chem 289: 8839-51 (2014)
Article DOI: 10.1074/jbc.M113.534859
BindingDB Entry DOI: 10.7270/Q2QZ28TM |
More data for this
Ligand-Target Pair | |
Androgen receptor [646-919]
(Homo sapiens (Human)) | BDBM227646
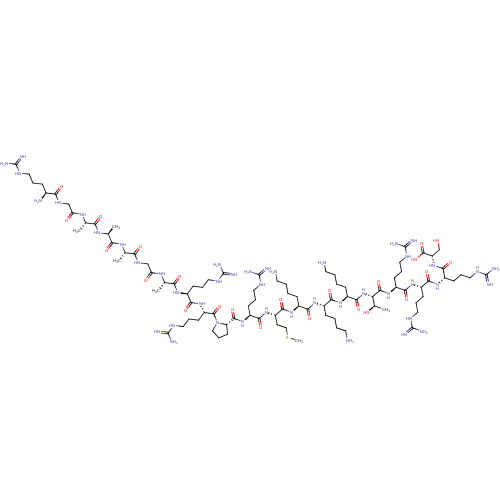
(Bag-1L (61-80) | RGAAAGARRPRMKKKTRRRS)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O |r| Show InChI InChI=1S/C93H176N44O23S/c1-48(123-71(144)51(4)124-70(143)49(2)121-67(141)46-120-73(146)53(97)21-13-36-112-87(98)99)69(142)119-45-66(140)122-50(3)72(145)125-57(25-14-37-113-88(100)101)79(152)134-63(30-19-42-118-93(110)111)85(158)137-43-20-31-65(137)83(156)132-60(28-17-40-116-91(106)107)77(150)131-62(32-44-161-6)81(154)127-55(23-8-11-34-95)74(147)126-54(22-7-10-33-94)75(148)128-56(24-9-12-35-96)82(155)136-68(52(5)139)84(157)133-61(29-18-41-117-92(108)109)78(151)129-58(26-15-38-114-89(102)103)76(149)130-59(27-16-39-115-90(104)105)80(153)135-64(47-138)86(159)160/h48-65,68,138-139H,7-47,94-97H2,1-6H3,(H,119,142)(H,120,146)(H,121,141)(H,122,140)(H,123,144)(H,124,143)(H,125,145)(H,126,147)(H,127,154)(H,128,148)(H,129,151)(H,130,149)(H,131,150)(H,132,156)(H,133,157)(H,134,152)(H,135,153)(H,136,155)(H,159,160)(H4,98,99,112)(H4,100,101,113)(H4,102,103,114)(H4,104,105,115)(H4,106,107,116)(H4,108,109,117)(H4,110,111,118)/t48-,49-,50-,51-,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,68-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Karlsruhe Institute of Technology
| Assay Description
We performed a mammalian one-hybrid assay in HeLa cells, where we transfected a construct coding for a Gal4DBD-BagN128 fusion or just Gal4DBD alone t... |
J Biol Chem 289: 8839-51 (2014)
Article DOI: 10.1074/jbc.M113.534859
BindingDB Entry DOI: 10.7270/Q2QZ28TM |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149
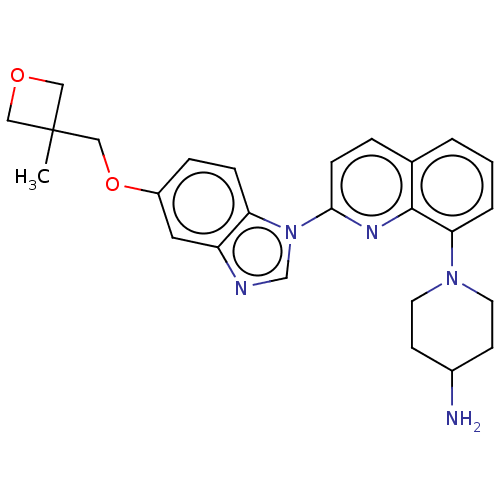
(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to non-auto-inhibited form of wild type human N-terminal GST-tagged FLT3 expressed in baculovirus expression |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149

(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 D835H mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50326053
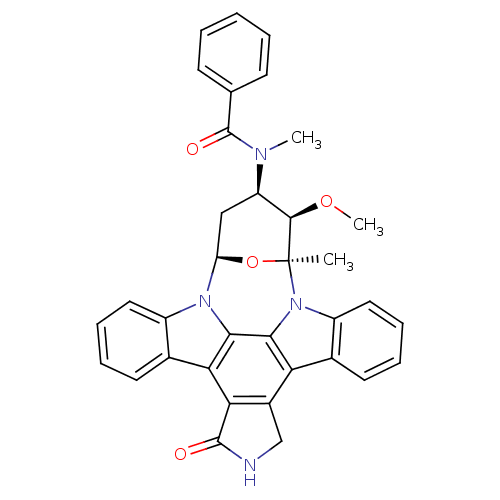
(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 D835H mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 D835H mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50526216

(CHEMBL4553725)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(NS(=O)(=O)C=C)cc23)c1C Show InChI InChI=1S/C24H31N5O4S/c1-6-29(7-2)12-11-25-24(31)22-15(4)21(26-16(22)5)14-19-18-13-17(28-34(32,33)8-3)9-10-20(18)27-23(19)30/h8-10,13-14,26,28H,3,6-7,11-12H2,1-2,4-5H3,(H,25,31)(H,27,30)/b19-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149

(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50526216

(CHEMBL4553725)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(NS(=O)(=O)C=C)cc23)c1C Show InChI InChI=1S/C24H31N5O4S/c1-6-29(7-2)12-11-25-24(31)22-15(4)21(26-16(22)5)14-19-18-13-17(28-34(32,33)8-3)9-10-20(18)27-23(19)30/h8-10,13-14,26,28H,3,6-7,11-12H2,1-2,4-5H3,(H,25,31)(H,27,30)/b19-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD/D835V double mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD/D835V double mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149

(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD/F691L double mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD/F691L double mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to non-auto-inhibited form of wild type human N-terminal GST-tagged FLT3 expressed in baculovirus expression |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to non-auto-inhibited form of wild type human N-terminal GST-tagged FLT3 expressed in baculovirus expression |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to non-auto-inhibited form of wild type human N-terminal GST-tagged FLT3 expressed in baculovirus expression |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50526216

(CHEMBL4553725)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(NS(=O)(=O)C=C)cc23)c1C Show InChI InChI=1S/C24H31N5O4S/c1-6-29(7-2)12-11-25-24(31)22-15(4)21(26-16(22)5)14-19-18-13-17(28-34(32,33)8-3)9-10-20(18)27-23(19)30/h8-10,13-14,26,28H,3,6-7,11-12H2,1-2,4-5H3,(H,25,31)(H,27,30)/b19-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to auto-inhibited form of wild type human N-terminal GST-tagged FLT3 expressed in baculovirus expression |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 D835H mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to non-auto-inhibited form of wild type human N-terminal GST-tagged FLT3 expressed in baculovirus expression |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to non-auto-inhibited form of wild type human N-terminal GST-tagged FLT3 expressed in baculovirus expression |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50526216

(CHEMBL4553725)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(NS(=O)(=O)C=C)cc23)c1C Show InChI InChI=1S/C24H31N5O4S/c1-6-29(7-2)12-11-25-24(31)22-15(4)21(26-16(22)5)14-19-18-13-17(28-34(32,33)8-3)9-10-20(18)27-23(19)30/h8-10,13-14,26,28H,3,6-7,11-12H2,1-2,4-5H3,(H,25,31)(H,27,30)/b19-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 D835H mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 D835H mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50526216

(CHEMBL4553725)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(NS(=O)(=O)C=C)cc23)c1C Show InChI InChI=1S/C24H31N5O4S/c1-6-29(7-2)12-11-25-24(31)22-15(4)21(26-16(22)5)14-19-18-13-17(28-34(32,33)8-3)9-10-20(18)27-23(19)30/h8-10,13-14,26,28H,3,6-7,11-12H2,1-2,4-5H3,(H,25,31)(H,27,30)/b19-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD/F691L double mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50526216

(CHEMBL4553725)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(NS(=O)(=O)C=C)cc23)c1C Show InChI InChI=1S/C24H31N5O4S/c1-6-29(7-2)12-11-25-24(31)22-15(4)21(26-16(22)5)14-19-18-13-17(28-34(32,33)8-3)9-10-20(18)27-23(19)30/h8-10,13-14,26,28H,3,6-7,11-12H2,1-2,4-5H3,(H,25,31)(H,27,30)/b19-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to non-auto-inhibited form of wild type human N-terminal GST-tagged FLT3 expressed in baculovirus expression |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 ITD mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 D835H mutant expressed in baculovirus expression system |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Androgen receptor [646-919]
(Homo sapiens (Human)) | BDBM227647
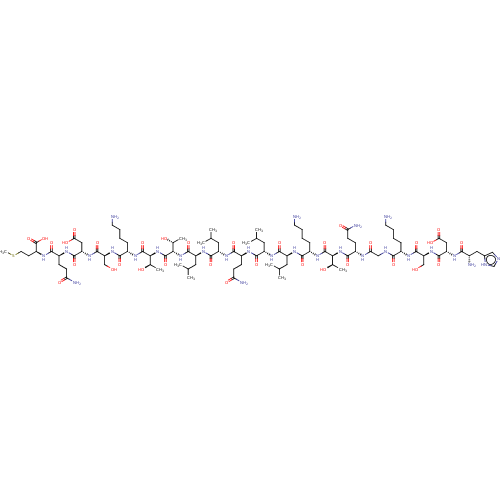
(HDSKGQTKLLQLLTTKSDQM | SCR-2 peptide)Show SMILES CSCC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)[C@@H](C)O)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | 6.5 | n/a |
Karlsruhe Institute of Technology
| Assay Description
For the fluorescence polarization experiments, 5 nM fluorescently labeled Bag-1L (Bag-1L(1-20), fluorescein isothiocyanate (FITC)-MAQRGGARRPRGDRERLGS... |
J Biol Chem 289: 8839-51 (2014)
Article DOI: 10.1074/jbc.M113.534859
BindingDB Entry DOI: 10.7270/Q2QZ28TM |
More data for this
Ligand-Target Pair | |
Androgen receptor [646-919]
(Homo sapiens (Human)) | BDBM227645

(Bag-1L (1-20) | MAQRGGARRPRGDRERLGSR)Show SMILES CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C89H160N42O27S/c1-43(2)36-56(72(147)116-41-64(137)120-58(42-132)79(154)129-55(82(157)158)20-12-33-112-89(104)105)130-75(150)51(18-10-31-110-87(100)101)124-77(152)53(23-25-65(138)139)126-73(148)50(17-9-30-109-86(98)99)125-78(153)57(37-66(140)141)119-63(136)40-115-71(146)48(15-7-28-107-84(94)95)127-80(155)59-21-13-34-131(59)81(156)54(19-11-32-111-88(102)103)128-74(149)49(16-8-29-108-85(96)97)121-67(142)44(3)117-62(135)39-113-61(134)38-114-70(145)47(14-6-27-106-83(92)93)123-76(151)52(22-24-60(91)133)122-68(143)45(4)118-69(144)46(90)26-35-159-5/h43-59,132H,6-42,90H2,1-5H3,(H2,91,133)(H,113,134)(H,114,145)(H,115,146)(H,116,147)(H,117,135)(H,118,144)(H,119,136)(H,120,137)(H,121,142)(H,122,143)(H,123,151)(H,124,152)(H,125,153)(H,126,148)(H,127,155)(H,128,149)(H,129,154)(H,130,150)(H,138,139)(H,140,141)(H,157,158)(H4,92,93,106)(H4,94,95,107)(H4,96,97,108)(H4,98,99,109)(H4,100,101,110)(H4,102,103,111)(H4,104,105,112)/t44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | 6.5 | n/a |
Karlsruhe Institute of Technology
| Assay Description
For the fluorescence polarization experiments, 5 nM fluorescently labeled Bag-1L (Bag-1L(1-20), fluorescein isothiocyanate (FITC)-MAQRGGARRPRGDRERLGS... |
J Biol Chem 289: 8839-51 (2014)
Article DOI: 10.1074/jbc.M113.534859
BindingDB Entry DOI: 10.7270/Q2QZ28TM |
More data for this
Ligand-Target Pair | |
Androgen receptor [646-919]
(Homo sapiens (Human)) | BDBM227646

(Bag-1L (61-80) | RGAAAGARRPRMKKKTRRRS)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O |r| Show InChI InChI=1S/C93H176N44O23S/c1-48(123-71(144)51(4)124-70(143)49(2)121-67(141)46-120-73(146)53(97)21-13-36-112-87(98)99)69(142)119-45-66(140)122-50(3)72(145)125-57(25-14-37-113-88(100)101)79(152)134-63(30-19-42-118-93(110)111)85(158)137-43-20-31-65(137)83(156)132-60(28-17-40-116-91(106)107)77(150)131-62(32-44-161-6)81(154)127-55(23-8-11-34-95)74(147)126-54(22-7-10-33-94)75(148)128-56(24-9-12-35-96)82(155)136-68(52(5)139)84(157)133-61(29-18-41-117-92(108)109)78(151)129-58(26-15-38-114-89(102)103)76(149)130-59(27-16-39-115-90(104)105)80(153)135-64(47-138)86(159)160/h48-65,68,138-139H,7-47,94-97H2,1-6H3,(H,119,142)(H,120,146)(H,121,141)(H,122,140)(H,123,144)(H,124,143)(H,125,145)(H,126,147)(H,127,154)(H,128,148)(H,129,151)(H,130,149)(H,131,150)(H,132,156)(H,133,157)(H,134,152)(H,135,153)(H,136,155)(H,159,160)(H4,98,99,112)(H4,100,101,113)(H4,102,103,114)(H4,104,105,115)(H4,106,107,116)(H4,108,109,117)(H4,110,111,118)/t48-,49-,50-,51-,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,68-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | 6.5 | n/a |
Karlsruhe Institute of Technology
| Assay Description
For the fluorescence polarization experiments, 5 nM fluorescently labeled Bag-1L (Bag-1L(1-20), fluorescein isothiocyanate (FITC)-MAQRGGARRPRGDRERLGS... |
J Biol Chem 289: 8839-51 (2014)
Article DOI: 10.1074/jbc.M113.534859
BindingDB Entry DOI: 10.7270/Q2QZ28TM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data