

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25826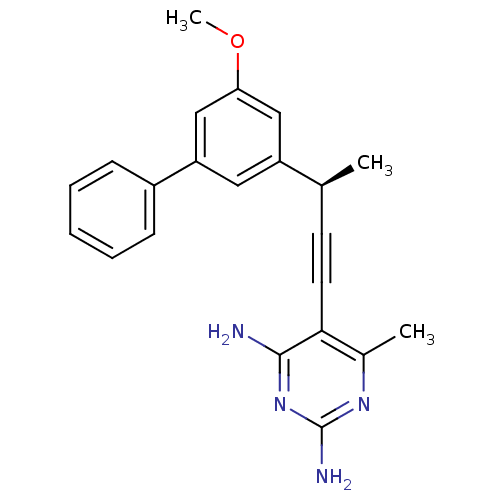 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25818 (5-[3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]-6-me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25819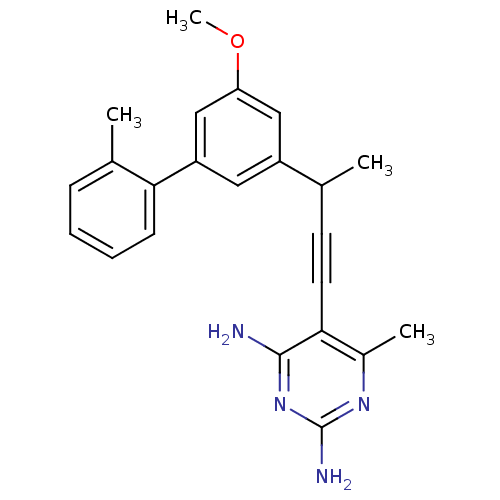 (5-{3-[3-methoxy-5-(2-methylphenyl)phenyl]but-1-yn-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25820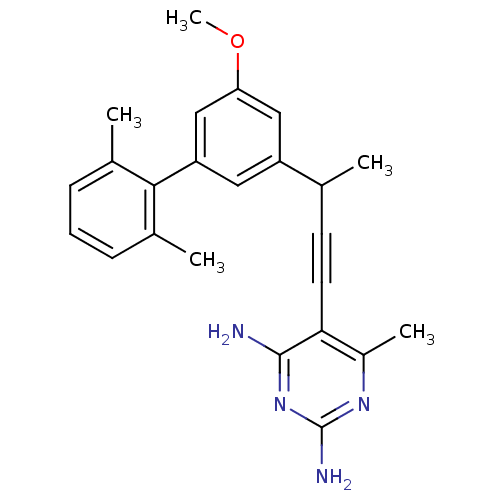 (5-{3-[3-(2,6-dimethylphenyl)-5-methoxyphenyl]but-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50329610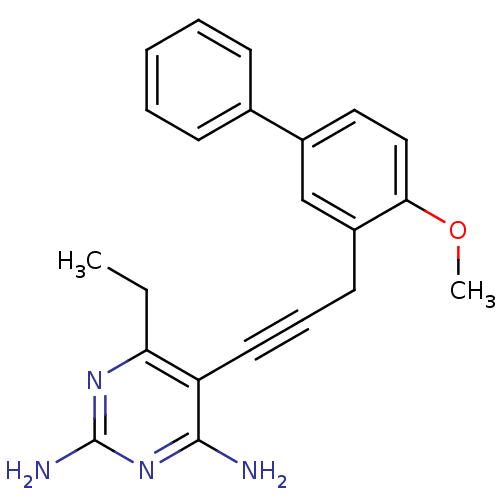 (6-ethyl-5-(3-(4-methoxybiphenyl-3-yl)prop-1-ynyl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50538416 (CHEMBL4644835 | US11236045, Example 25) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Ltd. Curated by ChEMBL | Assay Description Inhibition of NLRP3 inflammasome activation in LPS-primed human THP1 cells assessed as reduction in IL-1beta level preincubated for 1 hr followed by ... | ACS Med Chem Lett 11: 414-418 (2020) Article DOI: 10.1021/acsmedchemlett.9b00433 BindingDB Entry DOI: 10.7270/Q2BR8WP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM50329610 (6-ethyl-5-(3-(4-methoxybiphenyl-3-yl)prop-1-ynyl)p...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50538393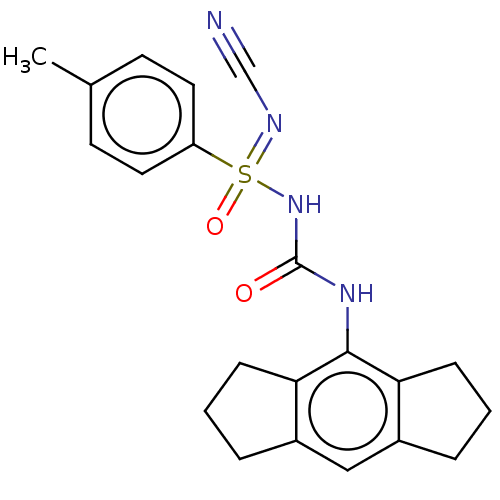 (CHEMBL4647321 | US11236045, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Ltd. Curated by ChEMBL | Assay Description Inhibition of NLRP3 inflammasome activation in LPS-primed human THP1 cells assessed as reduction in IL-1beta level preincubated for 1 hr followed by ... | ACS Med Chem Lett 11: 414-418 (2020) Article DOI: 10.1021/acsmedchemlett.9b00433 BindingDB Entry DOI: 10.7270/Q2BR8WP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM134283 (US8853228, F26M) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50155926 (CHEMBL3183703) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of NLRP3 in human THP1 cells assessed as inhibition of IL-1beta production | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127571 BindingDB Entry DOI: 10.7270/Q2SQ9402 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50155926 (CHEMBL3183703) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Ltd. Curated by ChEMBL | Assay Description Inhibition of NLRP3 inflammasome activation in LPS-primed human THP1 cells assessed as reduction in IL-1beta level preincubated for 1 hr followed by ... | ACS Med Chem Lett 11: 414-418 (2020) Article DOI: 10.1021/acsmedchemlett.9b00433 BindingDB Entry DOI: 10.7270/Q2BR8WP2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25821 (5-(3-{3-[2,6-bis(propan-2-yl)phenyl]-5-methoxyphen...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM15234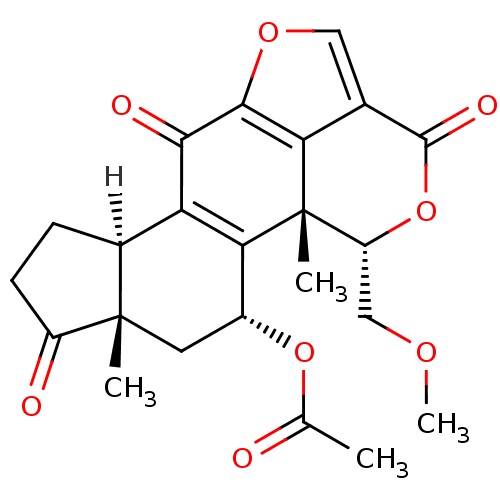 ((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant P110alpha/p85alpha using phosphatidyl inositol as substrate after 1 hr by ADP-Glo Kinase assay | Bioorg Med Chem Lett 22: 6919-22 (2012) Article DOI: 10.1016/j.bmcl.2012.09.015 BindingDB Entry DOI: 10.7270/Q2MK6F0Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134287 (US8853228, 150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50538418 (CHEMBL4641961 | US11236045, Example 54) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Ltd. Curated by ChEMBL | Assay Description Inhibition of NLRP3 inflammasome activation in LPS-primed human THP1 cells assessed as reduction in IL-1beta level preincubated for 1 hr followed by ... | ACS Med Chem Lett 11: 414-418 (2020) Article DOI: 10.1021/acsmedchemlett.9b00433 BindingDB Entry DOI: 10.7270/Q2BR8WP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134287 (US8853228, 150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50538405 (CHEMBL4642066) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Ltd. Curated by ChEMBL | Assay Description Inhibition of NLRP3 inflammasome activation in LPS-primed human THP1 cells assessed as reduction in IL-1beta level preincubated for 1 hr followed by ... | ACS Med Chem Lett 11: 414-418 (2020) Article DOI: 10.1021/acsmedchemlett.9b00433 BindingDB Entry DOI: 10.7270/Q2BR8WP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM134284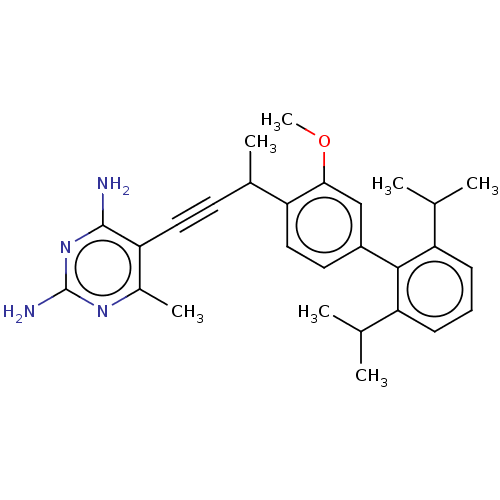 (US8853228, F26I) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50429695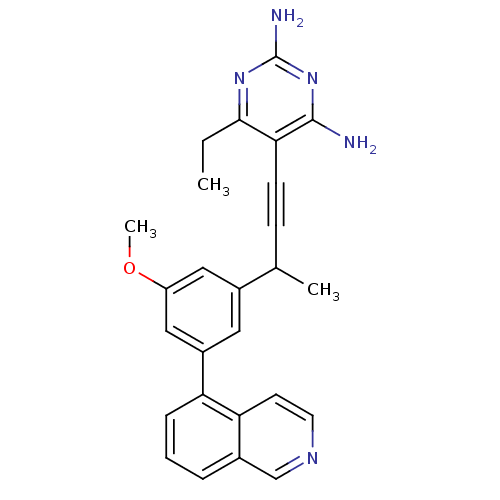 (CHEMBL2335421) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Candida albicans DHFR | Bioorg Med Chem Lett 23: 1279-84 (2013) Article DOI: 10.1016/j.bmcl.2013.01.008 BindingDB Entry DOI: 10.7270/Q2RV0Q2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50007898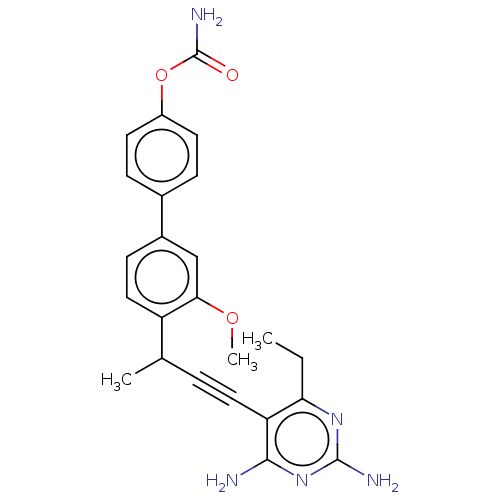 (CHEMBL3234317) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Candida albicans DHFR using dihydrofolate as substrate preincubated for 5 mins followed by substrate addition in presence of NADPH | J Med Chem 57: 2643-56 (2014) Article DOI: 10.1021/jm401916j BindingDB Entry DOI: 10.7270/Q26D5VJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429697 (CHEMBL2335419) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM50329607 (5-(3-(3-methoxybiphenyl-4-yl)but-1-ynyl)-6-methylp...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134286 (US8853228, 149) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429697 (CHEMBL2335419) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134288 (US8853228, 151) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50329607 (5-(3-(3-methoxybiphenyl-4-yl)but-1-ynyl)-6-methylp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Candida albicans DHFR using dihydrofolate as substrate preincubated for 5 mins followed by substrate addition in presence of NADPH | J Med Chem 57: 2643-56 (2014) Article DOI: 10.1021/jm401916j BindingDB Entry DOI: 10.7270/Q26D5VJ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50538407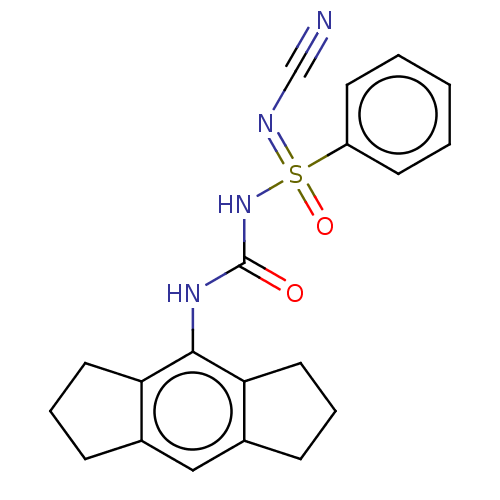 (CHEMBL4647621 | US11236045, Example 22) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Ltd. Curated by ChEMBL | Assay Description Inhibition of NLRP3 inflammasome activation in LPS-primed human THP1 cells assessed as reduction in IL-1beta level preincubated for 1 hr followed by ... | ACS Med Chem Lett 11: 414-418 (2020) Article DOI: 10.1021/acsmedchemlett.9b00433 BindingDB Entry DOI: 10.7270/Q2BR8WP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50429700 (CHEMBL2335416) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Candida albicans DHFR | Bioorg Med Chem Lett 23: 1279-84 (2013) Article DOI: 10.1016/j.bmcl.2013.01.008 BindingDB Entry DOI: 10.7270/Q2RV0Q2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134286 (US8853228, 149) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134286 (US8853228, 149) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50298800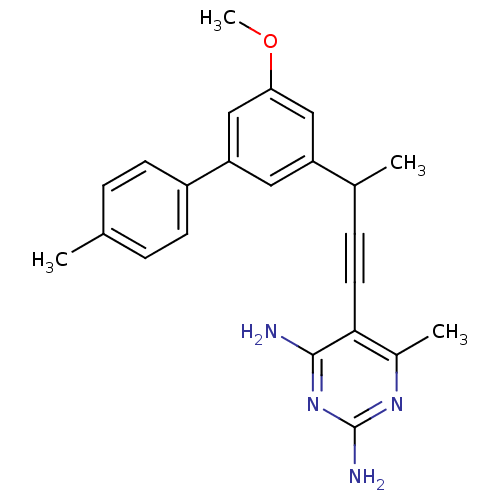 ((+/-)-5-(3-(5-methoxy-4'-methylbiphenyl-3-yl)but-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Candida albicans DHFR | Bioorg Med Chem Lett 23: 1279-84 (2013) Article DOI: 10.1016/j.bmcl.2013.01.008 BindingDB Entry DOI: 10.7270/Q2RV0Q2N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM50007817 (CHEMBL3234115) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134290 (US8853228, 155) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50007902 (CHEMBL3234321) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Candida albicans DHFR using dihydrofolate as substrate preincubated for 5 mins followed by substrate addition in presence of NADPH | J Med Chem 57: 2643-56 (2014) Article DOI: 10.1021/jm401916j BindingDB Entry DOI: 10.7270/Q26D5VJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50429693 (CHEMBL2335423) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Candida albicans DHFR | Bioorg Med Chem Lett 23: 1279-84 (2013) Article DOI: 10.1016/j.bmcl.2013.01.008 BindingDB Entry DOI: 10.7270/Q2RV0Q2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50429699 (CHEMBL2335417) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Candida albicans DHFR | Bioorg Med Chem Lett 23: 1279-84 (2013) Article DOI: 10.1016/j.bmcl.2013.01.008 BindingDB Entry DOI: 10.7270/Q2RV0Q2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50538421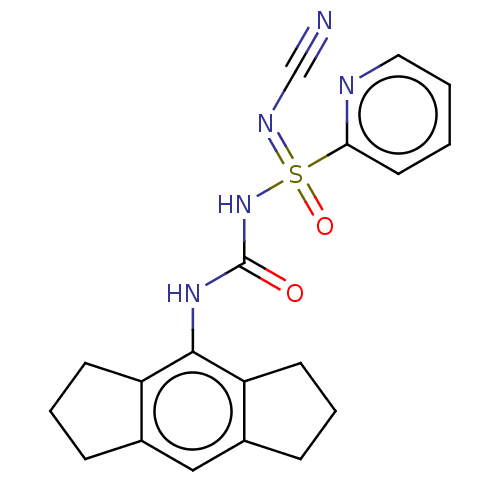 (CHEMBL4649141 | US11236045, Example 30) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Ltd. Curated by ChEMBL | Assay Description Inhibition of NLRP3 inflammasome activation in LPS-primed human THP1 cells assessed as reduction in IL-1beta level preincubated for 1 hr followed by ... | ACS Med Chem Lett 11: 414-418 (2020) Article DOI: 10.1021/acsmedchemlett.9b00433 BindingDB Entry DOI: 10.7270/Q2BR8WP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50547438 (CHEMBL4752523) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of NLRP3 inflammasome activation in human THP1 cells assessed as inhibition of IL-1beta release in presence of MSU | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127571 BindingDB Entry DOI: 10.7270/Q2SQ9402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429698 (CHEMBL2335418) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134285 (US8853228, 146) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM50429700 (CHEMBL2335416) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM50329609 (5-(3-(biphenyl-3-yl)prop-1-ynyl)-6-ethylpyrimidine...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429698 (CHEMBL2335418) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM50547438 (CHEMBL4752523) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of NLRP3 inflammasome activation in human THP1 cells assessed as inhibition of IL-1beta release in presence of nigericin | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127571 BindingDB Entry DOI: 10.7270/Q2SQ9402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429699 (CHEMBL2335417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134287 (US8853228, 150) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50329609 (5-(3-(biphenyl-3-yl)prop-1-ynyl)-6-ethylpyrimidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429700 (CHEMBL2335416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429700 (CHEMBL2335416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pyogenes) | BDBM134288 (US8853228, 151) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 209 total ) | Next | Last >> |