

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50561646 (CHEMBL4798102) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254203 (US10112937, Example 88 | US10150765, Example 88 | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 30 ... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM64682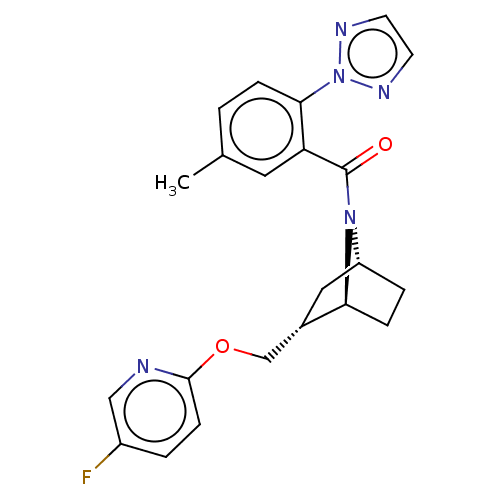 (US9475819, 10A) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409484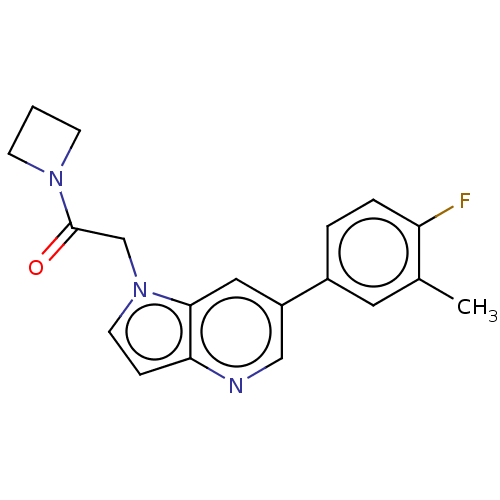 (1-(Azetidin-1-yl)-2-[6-(4-fluoro-3-methyl-phenyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409560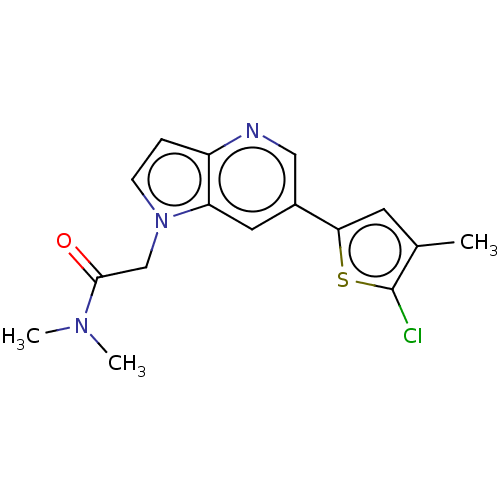 (2-[6-(5-Chloro-4-methyl-2-thienyl)pyrrolo[3,2-b]py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254266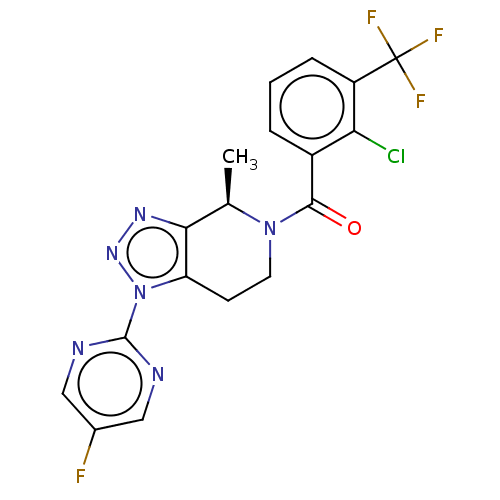 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Displacement of [3H]-A-804598 from recombinant human P2X7 expressed in human 1321N1 cells after 1 hr | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254266 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Displacement of [3H]-A-804598 from recombinant rat P2X7 expressed in human 1321N1 cells after 1 hr | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50509848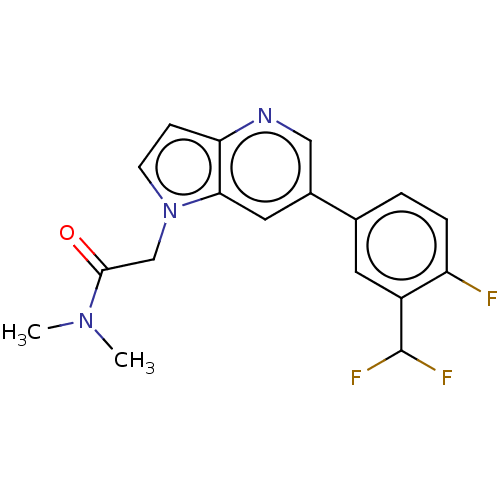 (CHEMBL4547576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50203731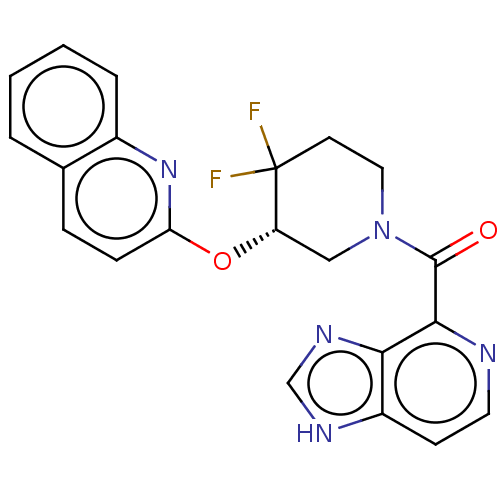 (CHEMBL3973097) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50438822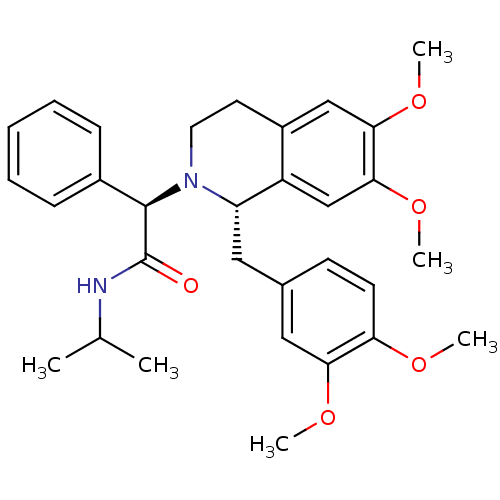 (CHEMBL2413367) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254160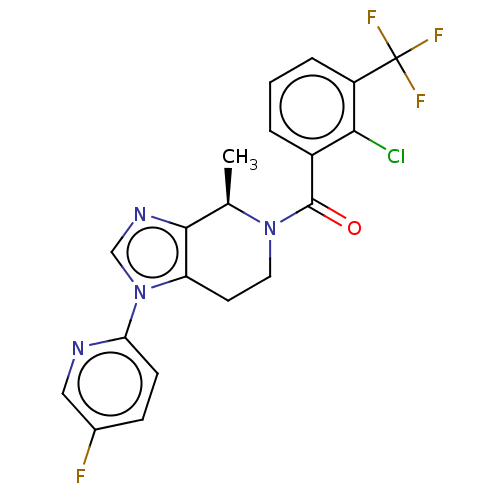 (US10112937, Example 40 | US10150765, Example 40 | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-(S)-7-(2-chloro-3-(trifluoromethyl)benzyl)-6-methyl-3-(pyrazin-2-yl)-6,7-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-8(5H)-one from re... | J Med Chem 59: 8535-48 (2016) Article DOI: 10.1021/acs.jmedchem.6b00989 BindingDB Entry DOI: 10.7270/Q28K7C1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254160 (US10112937, Example 40 | US10150765, Example 40 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-(S)-7-(2-chloro-3-(trifluoromethyl)benzyl)-6-methyl-3-(pyrazin-2-yl)-6,7-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-8(5H)-one from re... | J Med Chem 59: 8535-48 (2016) Article DOI: 10.1021/acs.jmedchem.6b00989 BindingDB Entry DOI: 10.7270/Q28K7C1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409128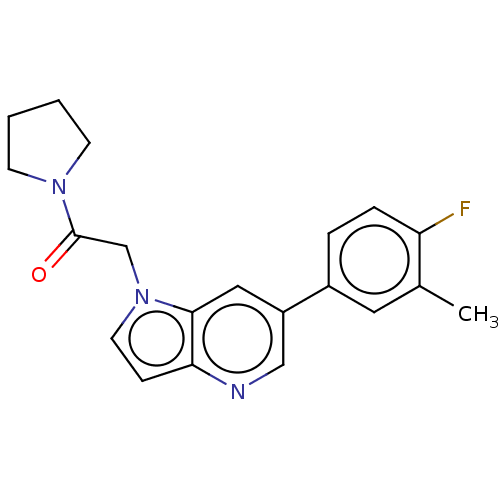 (2-[6-(4-Fluoro-3-methyl-phenyl)pyrrolo[3,2-b]pyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM163902 (US9062078, 33 | US9475819, 33 | US9637496, 33 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM65032 (US9475819, 279) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM471715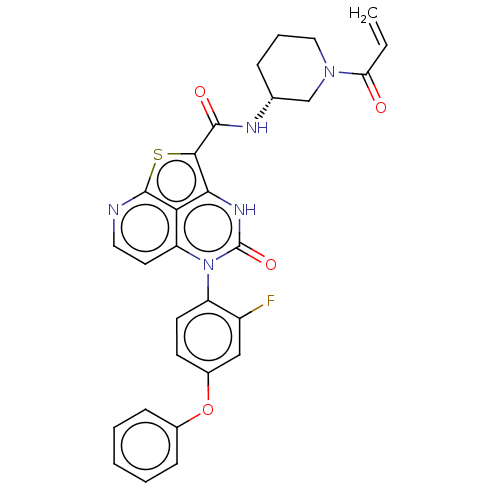 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-fluoro-4-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM163879 (US9062078, 11 | US9062078, 12A | US9062078, 12B | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM163876 (US9062078, 10A | US9062078, 10B | US9062078, 9 | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409482 (1-(3-Fluoroazetidin-1-yl)-2-[6-(4-fluoro-3-methyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409275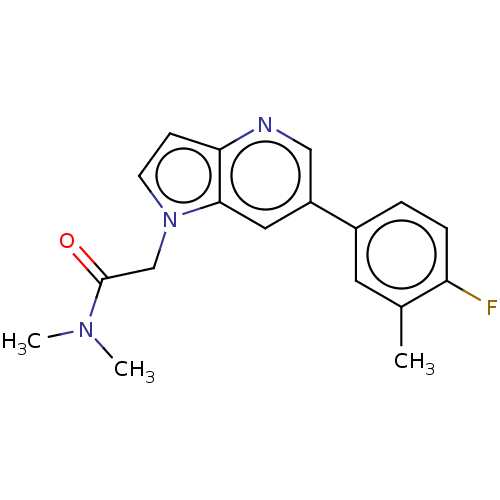 (2-[6-(4-Fluoro-3-methyl-phenyl)pyrrolo[3,2-b]pyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467364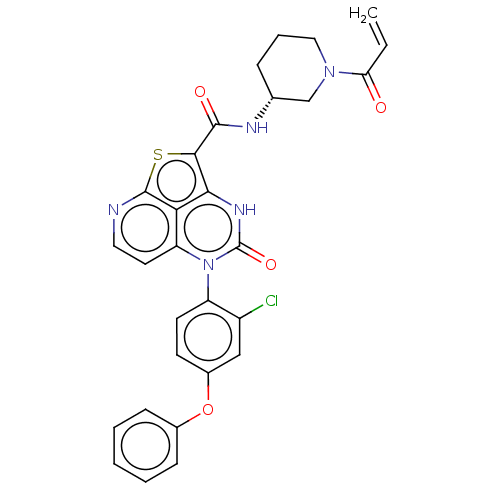 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-chloro-4-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50569791 (CHEMBL4846828) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409568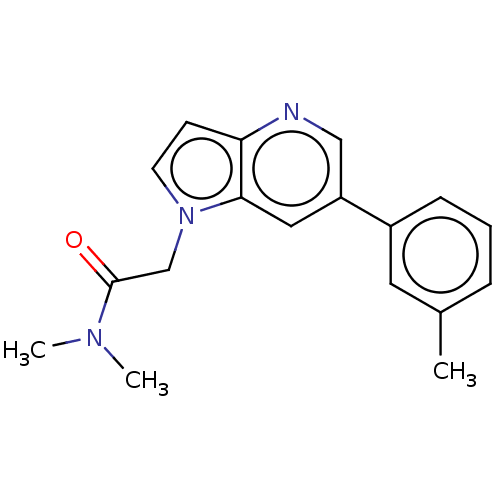 (N,N-Dimethyl-2-[6-(m-tolyl)pyrrolo[3,2-b]pyridin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254203 (US10112937, Example 88 | US10150765, Example 88 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 3... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM436689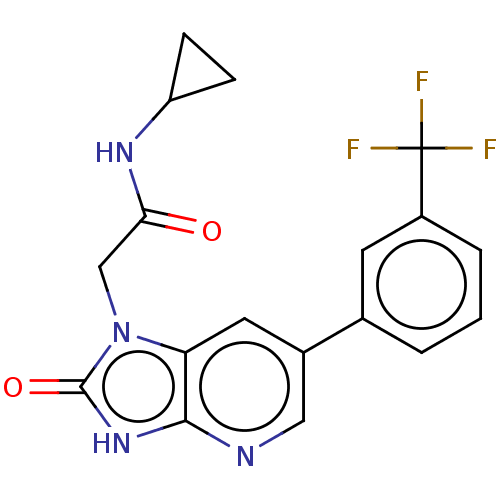 (N-Cyclopropyl-2-[2-oxo-6-[3-(trifluoromethyl)pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]Ro25-6981 from rat GluN2B receptor | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM163879 (US9062078, 11 | US9062078, 12A | US9062078, 12B | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OX2R | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM64646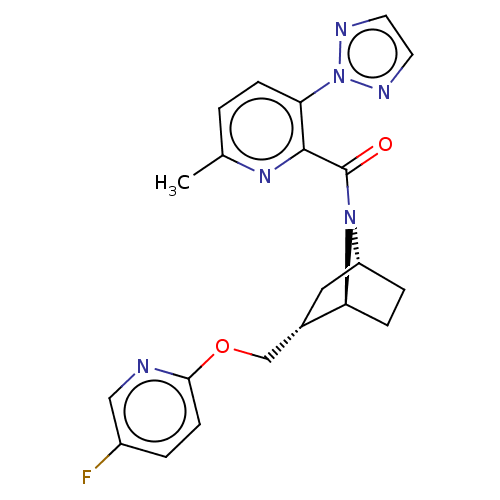 (US9475819, 8A) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50561653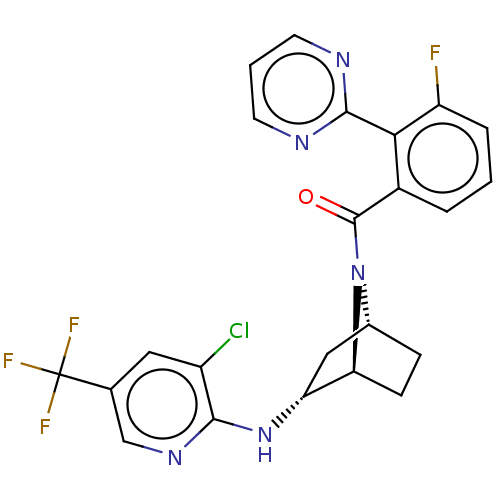 (CHEMBL4749384) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM485273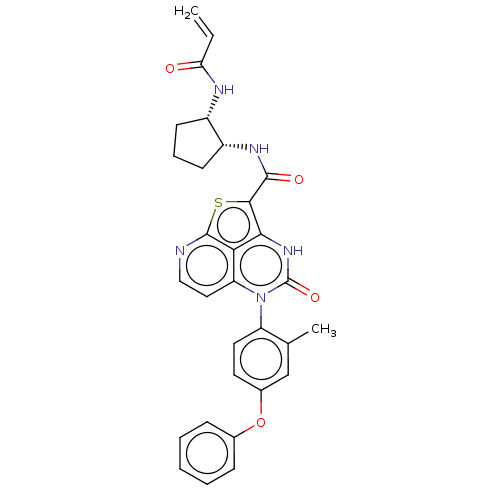 (N-((1R,2S)-2-Acrylamidocyclopentyl)-5-(*S)-(2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50561652 (CHEMBL4752792) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409569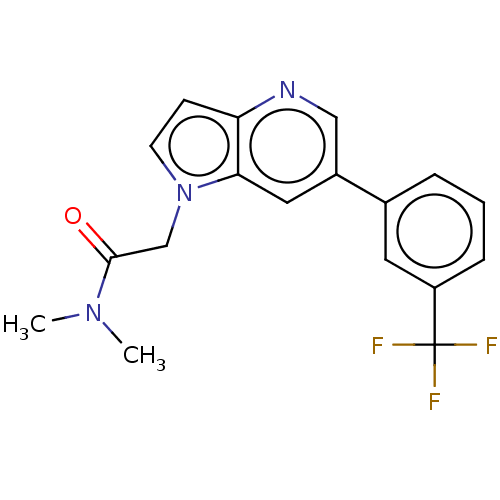 (N,N-Dimethyl-2-[6-[3-(trifluoromethyl)phenyl]pyrro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409264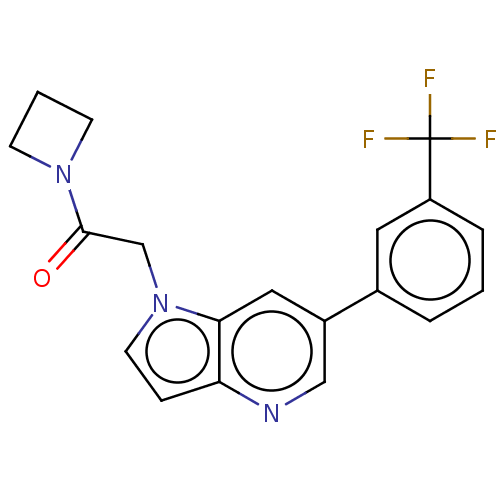 (1-(Azetidin-1-yl)-2-[6-[3-(trifluoromethyl)phenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409572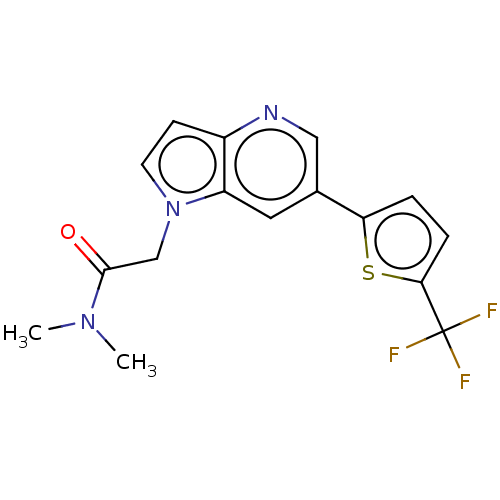 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50561651 (CHEMBL4786307) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM4535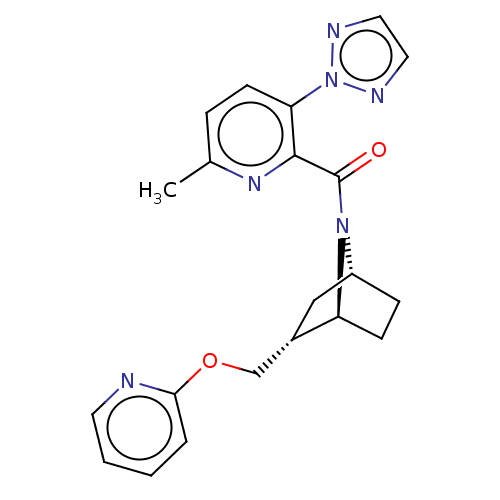 (US9475819, 5A) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50561647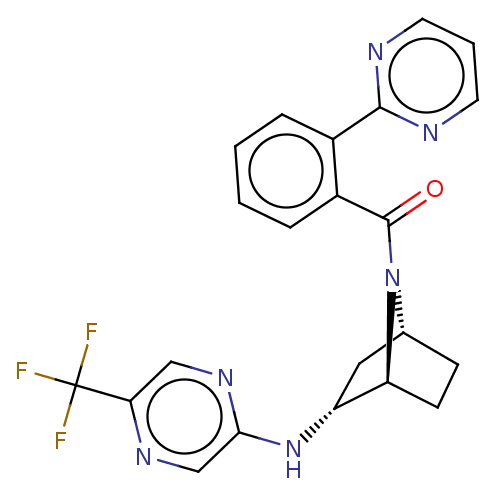 (CHEMBL4750440) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM4532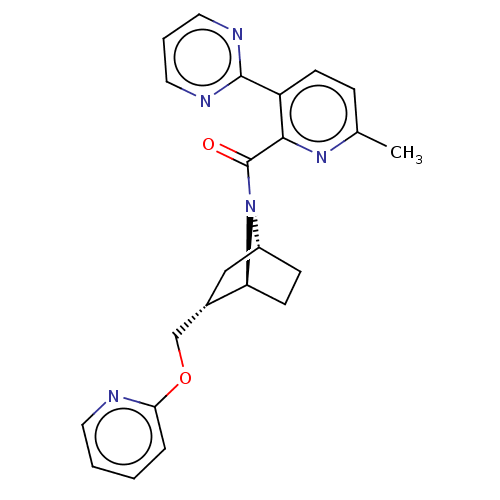 (US9475819, 3B) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM468103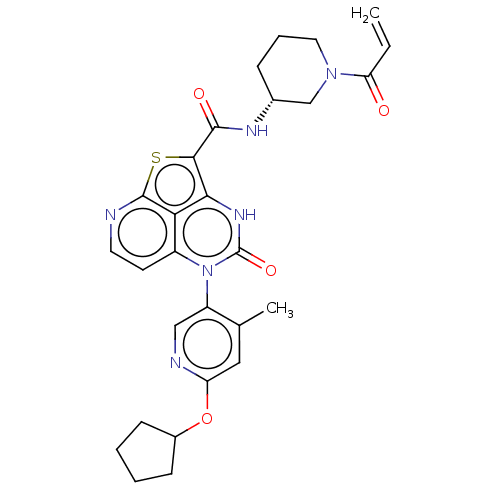 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-(cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254296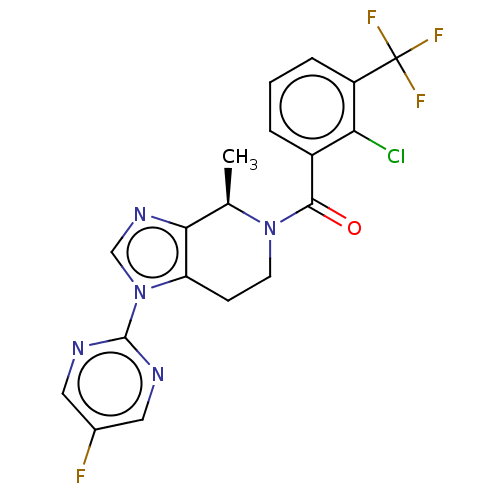 (US10112937, Example 193 | US10150765, Example 193 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-(S)-7-(2-chloro-3-(trifluoromethyl)benzyl)-6-methyl-3-(pyrazin-2-yl)-6,7-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-8(5H)-one from re... | J Med Chem 59: 8535-48 (2016) Article DOI: 10.1021/acs.jmedchem.6b00989 BindingDB Entry DOI: 10.7270/Q28K7C1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50561648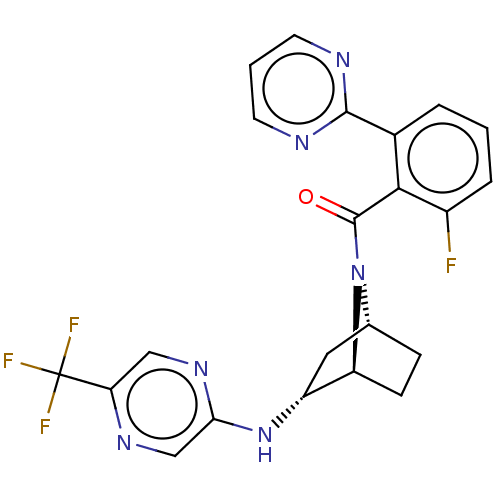 (CHEMBL4743991) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409602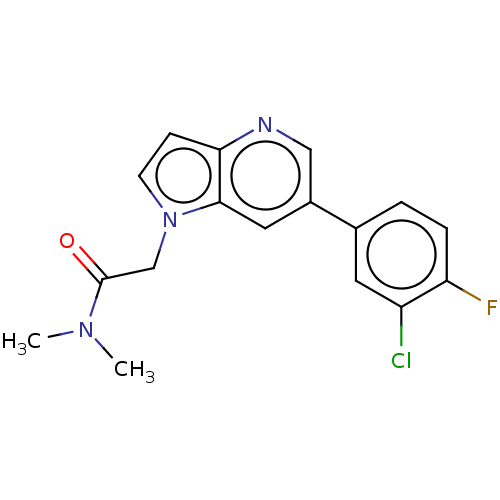 (2-[6-(3-Chloro-4-fluoro-phenyl)pyrrolo[3,2-b]pyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409513 (2-[6-(5-Chloro-2-thienyl)pyrrolo[3,2-b]pyridin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM468000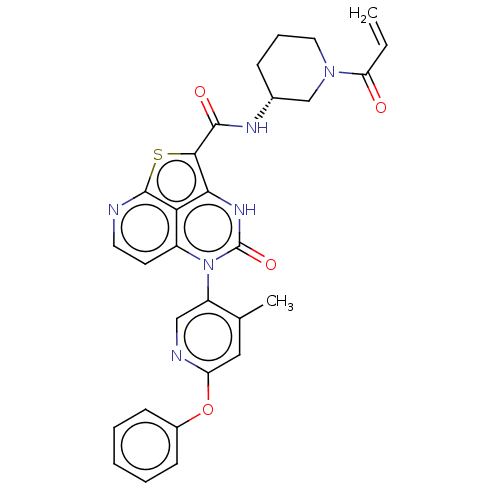 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(4-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467718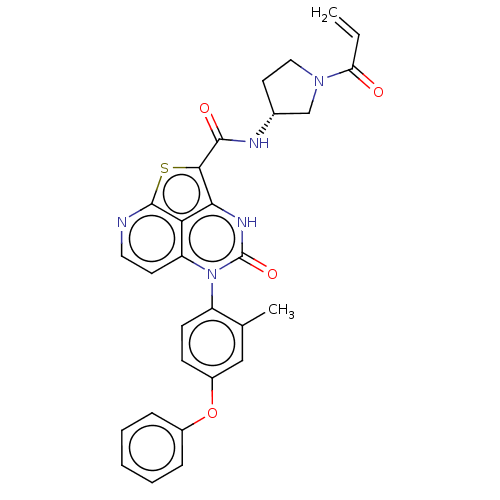 ((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467718 ((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM65030 (US9475819, 181) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OXIR | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM64682 (US9475819, 10A) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human OX2R | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00085 BindingDB Entry DOI: 10.7270/Q2348Q4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467367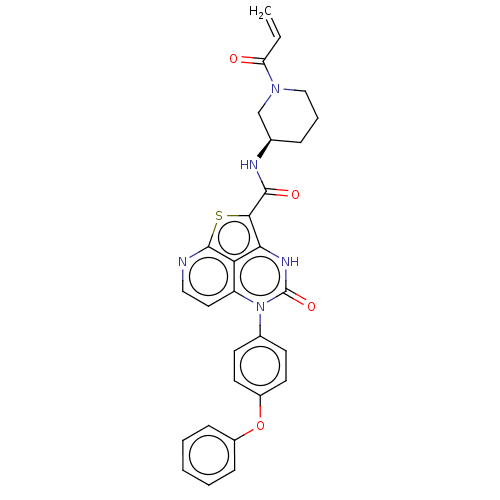 ((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(4-phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254164 (US10112937, Example 44 | US10150765, Example 44 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-(S)-7-(2-chloro-3-(trifluoromethyl)benzyl)-6-methyl-3-(pyrazin-2-yl)-6,7-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-8(5H)-one from re... | J Med Chem 59: 8535-48 (2016) Article DOI: 10.1021/acs.jmedchem.6b00989 BindingDB Entry DOI: 10.7270/Q28K7C1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM409301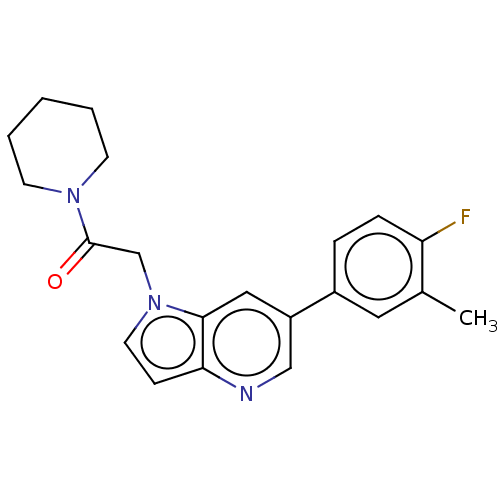 (2-[6-(4-Fluoro-3-methyl-phenyl)pyrrolo[3,2-b]pyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]-1-(azetidin-1-yl)-2-(6-(4-fluoro-3-methylphenyl)-1H-pyrrolo[3,2-b]pyridin-1-yl)ethanone from rat adult cortex GluN2B receptor me... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 542 total ) | Next | Last >> |