 Found 38 hits with Last Name = 'baxt' and Initial = 'l'
Found 38 hits with Last Name = 'baxt' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-1
(Homo sapiens (Human)) | BDBM50200932
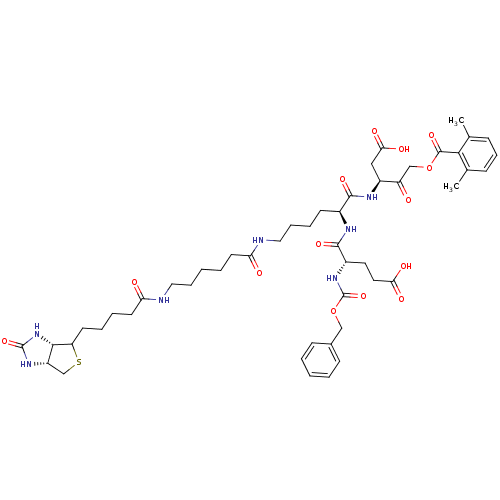
((3S)-3-[(2S)-6-(6-{5-[(3aS,6aR)-2-oxo-hexahydro-1H...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C49H67N7O14S/c1-30-14-13-15-31(2)43(30)47(66)69-28-37(57)35(26-42(62)63)53-45(64)33(52-46(65)34(22-23-41(60)61)55-49(68)70-27-32-16-5-3-6-17-32)18-10-12-25-51-39(58)20-7-4-11-24-50-40(59)21-9-8-19-38-44-36(29-71-38)54-48(67)56-44/h3,5-6,13-17,33-36,38,44H,4,7-12,18-29H2,1-2H3,(H,50,59)(H,51,58)(H,52,65)(H,53,64)(H,55,68)(H,60,61)(H,62,63)(H2,54,56,67)/t33-,34-,35-,36-,38?,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 1 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Caspase-8
(Homo sapiens (Human)) | BDBM50200932

((3S)-3-[(2S)-6-(6-{5-[(3aS,6aR)-2-oxo-hexahydro-1H...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C49H67N7O14S/c1-30-14-13-15-31(2)43(30)47(66)69-28-37(57)35(26-42(62)63)53-45(64)33(52-46(65)34(22-23-41(60)61)55-49(68)70-27-32-16-5-3-6-17-32)18-10-12-25-51-39(58)20-7-4-11-24-50-40(59)21-9-8-19-38-44-36(29-71-38)54-48(67)56-44/h3,5-6,13-17,33-36,38,44H,4,7-12,18-29H2,1-2H3,(H,50,59)(H,51,58)(H,52,65)(H,53,64)(H,55,68)(H,60,61)(H,62,63)(H2,54,56,67)/t33-,34-,35-,36-,38?,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 8 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50200932

((3S)-3-[(2S)-6-(6-{5-[(3aS,6aR)-2-oxo-hexahydro-1H...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C49H67N7O14S/c1-30-14-13-15-31(2)43(30)47(66)69-28-37(57)35(26-42(62)63)53-45(64)33(52-46(65)34(22-23-41(60)61)55-49(68)70-27-32-16-5-3-6-17-32)18-10-12-25-51-39(58)20-7-4-11-24-50-40(59)21-9-8-19-38-44-36(29-71-38)54-48(67)56-44/h3,5-6,13-17,33-36,38,44H,4,7-12,18-29H2,1-2H3,(H,50,59)(H,51,58)(H,52,65)(H,53,64)(H,55,68)(H,60,61)(H,62,63)(H2,54,56,67)/t33-,34-,35-,36-,38?,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 3 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50200934
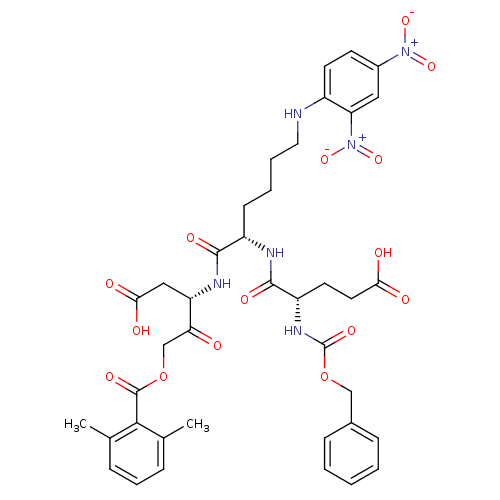
(2,6-dimethyl-benzoic acid (S)-3-[(S)-2-((S)-2-benz...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C39H44N6O15/c1-23-9-8-10-24(2)35(23)38(53)59-22-32(46)30(20-34(49)50)42-36(51)28(13-6-7-18-40-27-15-14-26(44(55)56)19-31(27)45(57)58)41-37(52)29(16-17-33(47)48)43-39(54)60-21-25-11-4-3-5-12-25/h3-5,8-12,14-15,19,28-30,40H,6-7,13,16-18,20-22H2,1-2H3,(H,41,52)(H,42,51)(H,43,54)(H,47,48)(H,49,50)/t28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 3 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50200935
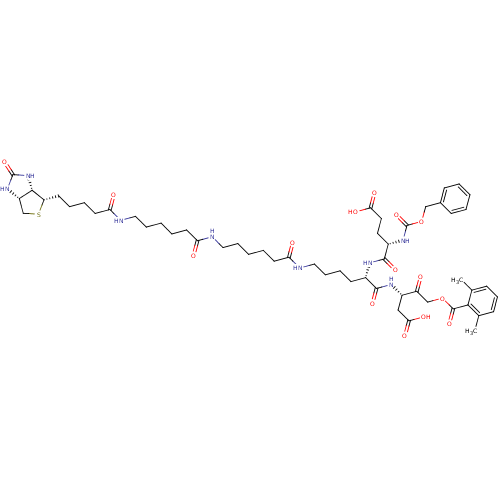
((3S)-3-[(2S)-6-[6-(6-{5-[(3aS,4S,6aR)-2-oxo-hexahy...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C55H78N8O15S/c1-35-17-16-18-36(2)49(35)53(74)77-33-42(64)40(31-48(70)71)60-51(72)38(59-52(73)39(26-27-47(68)69)62-55(76)78-32-37-19-6-3-7-20-37)21-12-15-30-58-45(66)24-9-5-13-28-56-44(65)23-8-4-14-29-57-46(67)25-11-10-22-43-50-41(34-79-43)61-54(75)63-50/h3,6-7,16-20,38-41,43,50H,4-5,8-15,21-34H2,1-2H3,(H,56,65)(H,57,67)(H,58,66)(H,59,73)(H,60,72)(H,62,76)(H,68,69)(H,70,71)(H2,61,63,75)/t38-,39-,40-,41-,43-,50-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 3 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Caspase-7
(Homo sapiens (Human)) | BDBM50200932

((3S)-3-[(2S)-6-(6-{5-[(3aS,6aR)-2-oxo-hexahydro-1H...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C49H67N7O14S/c1-30-14-13-15-31(2)43(30)47(66)69-28-37(57)35(26-42(62)63)53-45(64)33(52-46(65)34(22-23-41(60)61)55-49(68)70-27-32-16-5-3-6-17-32)18-10-12-25-51-39(58)20-7-4-11-24-50-40(59)21-9-8-19-38-44-36(29-71-38)54-48(67)56-44/h3,5-6,13-17,33-36,38,44H,4,7-12,18-29H2,1-2H3,(H,50,59)(H,51,58)(H,52,65)(H,53,64)(H,55,68)(H,60,61)(H,62,63)(H2,54,56,67)/t33-,34-,35-,36-,38?,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 7 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50200933
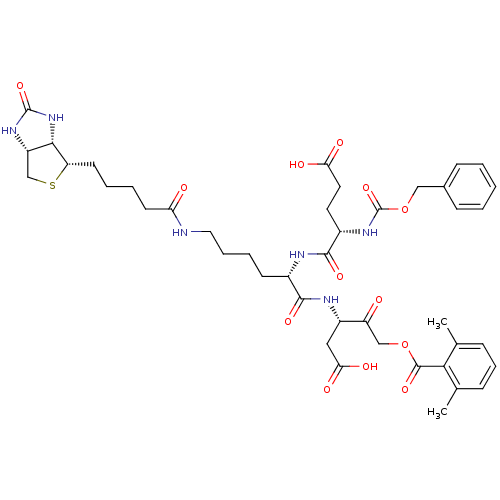
(2,6-dimethyl-benzoic acid (S)-3-{(S)-2-((S)-2-benz...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C43H56N6O13S/c1-25-11-10-12-26(2)37(25)41(58)61-23-32(50)30(21-36(54)55)46-39(56)28(45-40(57)29(18-19-35(52)53)48-43(60)62-22-27-13-4-3-5-14-27)15-8-9-20-44-34(51)17-7-6-16-33-38-31(24-63-33)47-42(59)49-38/h3-5,10-14,28-31,33,38H,6-9,15-24H2,1-2H3,(H,44,51)(H,45,57)(H,46,56)(H,48,60)(H,52,53)(H,54,55)(H2,47,49,59)/t28-,29-,30-,31-,33-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 3 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50200931
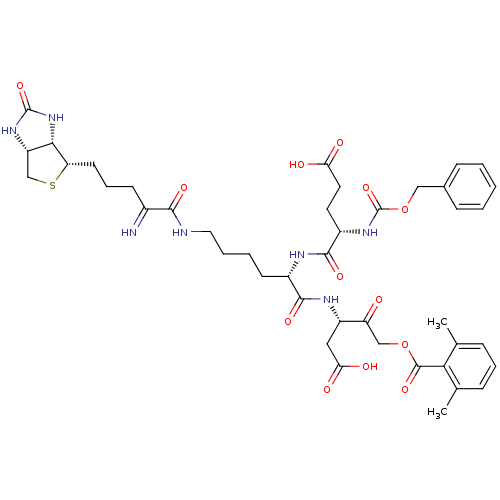
(2,6-dimethyl-benzoic acid (S)-3-{(S)-2-((S)-2-benz...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)C(=N)CCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C43H55N7O13S/c1-24-10-8-11-25(2)36(24)41(59)62-22-32(51)30(20-35(54)55)47-39(57)28(46-40(58)29(17-18-34(52)53)49-43(61)63-21-26-12-4-3-5-13-26)15-6-7-19-45-38(56)27(44)14-9-16-33-37-31(23-64-33)48-42(60)50-37/h3-5,8,10-13,28-31,33,37,44H,6-7,9,14-23H2,1-2H3,(H,45,56)(H,46,58)(H,47,57)(H,49,61)(H,52,53)(H,54,55)(H2,48,50,60)/t28-,29-,30-,31-,33-,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 3 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Caspase-2
(Homo sapiens (Human)) | BDBM50200932

((3S)-3-[(2S)-6-(6-{5-[(3aS,6aR)-2-oxo-hexahydro-1H...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C49H67N7O14S/c1-30-14-13-15-31(2)43(30)47(66)69-28-37(57)35(26-42(62)63)53-45(64)33(52-46(65)34(22-23-41(60)61)55-49(68)70-27-32-16-5-3-6-17-32)18-10-12-25-51-39(58)20-7-4-11-24-50-40(59)21-9-8-19-38-44-36(29-71-38)54-48(67)56-44/h3,5-6,13-17,33-36,38,44H,4,7-12,18-29H2,1-2H3,(H,50,59)(H,51,58)(H,52,65)(H,53,64)(H,55,68)(H,60,61)(H,62,63)(H2,54,56,67)/t33-,34-,35-,36-,38?,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 2 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Caspase-6
(Homo sapiens (Human)) | BDBM50200932

((3S)-3-[(2S)-6-(6-{5-[(3aS,6aR)-2-oxo-hexahydro-1H...)Show SMILES Cc1cccc(C)c1C(=O)OCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C49H67N7O14S/c1-30-14-13-15-31(2)43(30)47(66)69-28-37(57)35(26-42(62)63)53-45(64)33(52-46(65)34(22-23-41(60)61)55-49(68)70-27-32-16-5-3-6-17-32)18-10-12-25-51-39(58)20-7-4-11-24-50-40(59)21-9-8-19-38-44-36(29-71-38)54-48(67)56-44/h3,5-6,13-17,33-36,38,44H,4,7-12,18-29H2,1-2H3,(H,50,59)(H,51,58)(H,52,65)(H,53,64)(H,55,68)(H,60,61)(H,62,63)(H2,54,56,67)/t33-,34-,35-,36-,38?,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human caspase 6 |
J Med Chem 49: 7636-45 (2006)
Article DOI: 10.1021/jm060385h
BindingDB Entry DOI: 10.7270/Q2319VJT |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586159
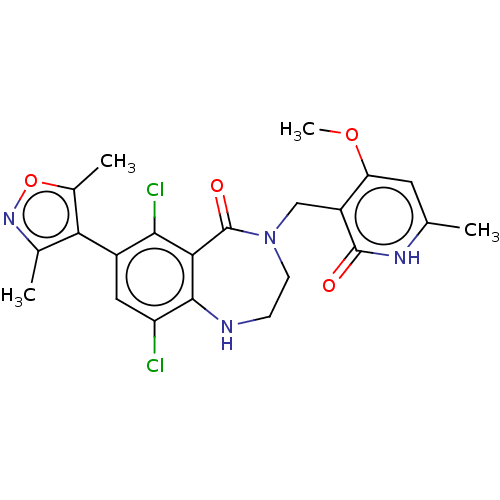
(CHEMBL5087917)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCNc2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.78,;2.93,-1.29,;4.02,-.2,;5.51,-.6,;6.6,.49,;8.09,.09,;6.2,1.98,;4.71,2.37,;4.31,3.86,;3.62,1.29,;2.14,1.68,;1.05,.6,;1.72,-.78,;1.05,-2.15,;-.45,-2.51,;-1.65,-1.55,;-2.98,-2.32,;-2.98,-3.86,;-4.32,-1.56,;-4.32,-.01,;-5.65,.76,;-7.06,.13,;-7.45,-1.36,;-8.09,1.27,;-7.32,2.61,;-5.81,2.29,;-4.72,3.38,;-2.98,.75,;-2.98,2.29,;-1.65,-.01,;-.46,.94,;-.85,2.42,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586160

(CHEMBL5085993)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.71,;2.93,-1.22,;4.02,-.13,;5.51,-.53,;6.6,.56,;8.09,.16,;6.2,2.05,;4.71,2.44,;4.31,3.93,;3.62,1.35,;2.14,1.75,;1.05,.66,;1.72,-.71,;1.05,-2.09,;-.45,-2.44,;-.85,-3.93,;-1.65,-1.48,;-2.98,-2.25,;-2.98,-3.79,;-4.32,-1.49,;-4.32,.05,;-5.65,.82,;-7.06,.2,;-7.45,-1.29,;-8.09,1.34,;-7.32,2.68,;-5.81,2.36,;-4.72,3.44,;-2.98,.82,;-2.98,2.36,;-1.65,.06,;-.46,1.01,;-.85,2.49,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586173

(CHEMBL5087318)Show SMILES CSc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.71,;2.93,-1.22,;4.02,-.13,;5.51,-.53,;6.6,.56,;8.09,.16,;6.2,2.05,;4.71,2.44,;4.31,3.93,;3.62,1.35,;2.14,1.75,;1.05,.66,;1.72,-.72,;1.05,-2.09,;-.45,-2.44,;-.85,-3.93,;-1.65,-1.48,;-2.98,-2.25,;-2.98,-3.79,;-4.32,-1.49,;-4.32,.05,;-5.65,.82,;-7.06,.2,;-7.45,-1.29,;-8.09,1.34,;-7.32,2.68,;-5.81,2.36,;-4.72,3.44,;-2.98,.82,;-2.98,2.36,;-1.65,.05,;-.46,1.01,;-.85,2.49,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709
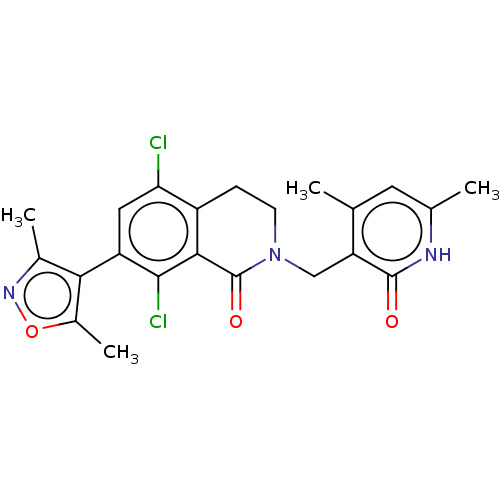
(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586158
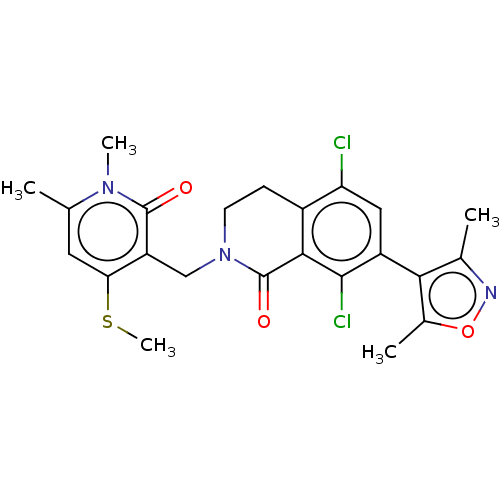
(CHEMBL5088217)Show SMILES CSc1cc(C)n(C)c(=O)c1CN1CCc2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(2.55,-3.62,;2.55,-2.08,;3.88,-1.31,;5.22,-2.08,;6.55,-1.31,;7.88,-2.08,;6.55,.23,;7.88,1,;5.22,1,;5.22,2.54,;3.88,.23,;2.55,1,;1.22,.23,;1.22,-1.31,;-.12,-2.08,;-1.45,-1.31,;-2.78,-2.08,;-2.78,-3.62,;-4.11,-1.32,;-4.11,.23,;-5.45,1,;-6.85,.37,;-7.25,-1.11,;-7.88,1.52,;-7.11,2.85,;-5.61,2.53,;-4.52,3.62,;-2.78,1,;-2.78,2.54,;-1.45,.23,;-.12,1,;-.12,2.54,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586171

(CHEMBL5071153)Show SMILES CCc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.71,;2.93,-1.22,;4.02,-.13,;5.51,-.53,;6.6,.56,;8.09,.16,;6.2,2.04,;4.71,2.44,;4.31,3.93,;3.62,1.35,;2.14,1.75,;1.05,.66,;1.72,-.72,;1.05,-2.09,;-.45,-2.44,;-.85,-3.93,;-1.65,-1.48,;-2.98,-2.25,;-2.98,-3.79,;-4.32,-1.49,;-4.32,.05,;-5.65,.82,;-7.06,.2,;-7.45,-1.29,;-8.09,1.34,;-7.32,2.68,;-5.81,2.36,;-4.72,3.44,;-2.98,.82,;-2.98,2.36,;-1.65,.05,;-.46,1.01,;-.85,2.49,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586167
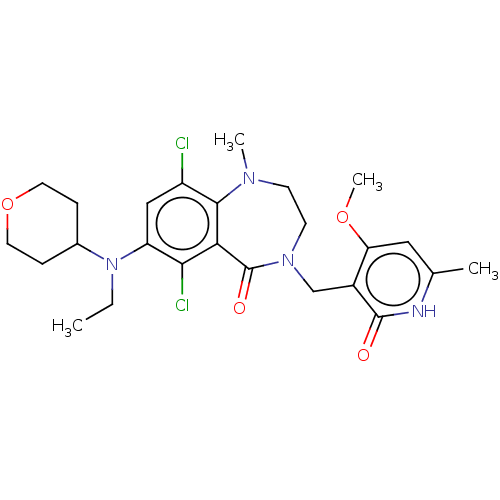
(CHEMBL5074786)Show SMILES CCN(C1CCOCC1)c1cc(Cl)c2N(C)CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586165

(CHEMBL5093442)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(C(C)C3CCOCC3)c(Cl)c2C1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586175

(CHEMBL5088474)Show SMILES CSc1cc(C)n(C)c(=O)c1CN1CCNc2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.78,;2.93,-1.29,;4.02,-.2,;5.51,-.6,;6.6,.49,;8.09,.09,;6.2,1.98,;7.29,3.07,;4.71,2.37,;4.31,3.86,;3.62,1.29,;2.14,1.68,;1.05,.6,;1.72,-.78,;1.05,-2.15,;-.45,-2.51,;-1.65,-1.55,;-2.98,-2.32,;-2.98,-3.86,;-4.32,-1.56,;-4.32,-.01,;-5.65,.76,;-7.06,.13,;-7.45,-1.36,;-8.09,1.27,;-7.32,2.61,;-5.81,2.29,;-4.72,3.38,;-2.98,.75,;-2.98,2.3,;-1.65,-.01,;-.46,.94,;-.85,2.42,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586162
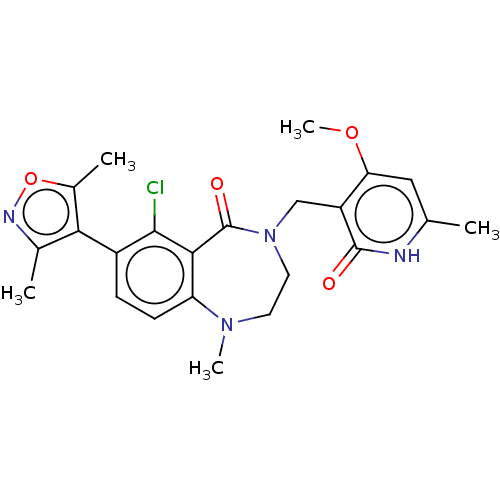
(CHEMBL5079717)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCN(C)c2ccc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.71,;2.93,-1.22,;4.02,-.13,;5.51,-.53,;6.6,.56,;8.09,.16,;6.2,2.05,;4.71,2.44,;4.31,3.93,;3.62,1.35,;2.14,1.75,;1.05,.66,;1.72,-.71,;1.05,-2.08,;-.45,-2.44,;-.85,-3.93,;-1.65,-1.48,;-2.98,-2.25,;-4.32,-1.49,;-4.32,.05,;-5.65,.82,;-7.06,.2,;-7.45,-1.29,;-8.09,1.34,;-7.32,2.68,;-5.81,2.36,;-4.72,3.44,;-2.98,.82,;-2.98,2.36,;-1.65,.06,;-.46,1.01,;-.85,2.49,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586166

(CHEMBL5091527)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(C(O)C3CCOCC3)c(Cl)c2C1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586174
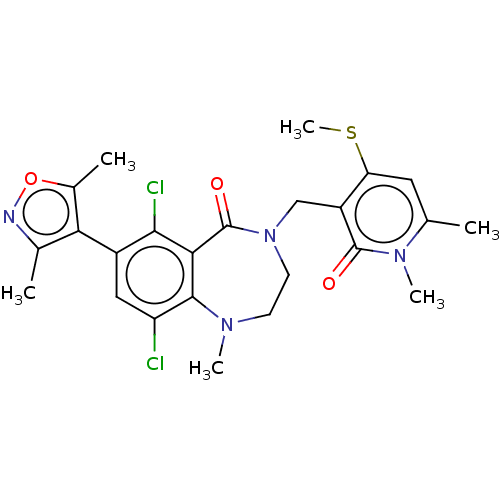
(CHEMBL5081019)Show SMILES CSc1cc(C)n(C)c(=O)c1CN1CCN(C)c2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.71,;2.93,-1.22,;4.02,-.13,;5.51,-.53,;6.6,.56,;8.09,.16,;6.2,2.05,;7.29,3.13,;4.71,2.44,;4.31,3.93,;3.62,1.35,;2.14,1.75,;1.05,.66,;1.72,-.72,;1.05,-2.09,;-.45,-2.44,;-.85,-3.93,;-1.65,-1.48,;-2.98,-2.25,;-2.98,-3.79,;-4.32,-1.49,;-4.32,.05,;-5.65,.82,;-7.06,.2,;-7.45,-1.29,;-8.09,1.34,;-7.32,2.68,;-5.81,2.36,;-4.72,3.44,;-2.98,.82,;-2.98,2.36,;-1.65,.05,;-.46,1.01,;-.85,2.49,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586157

(CHEMBL5082529)Show SMILES COc1cc(C)n(C)c(=O)c1CN1CCc2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(2.55,-3.62,;2.55,-2.08,;3.88,-1.31,;5.22,-2.08,;6.55,-1.31,;7.88,-2.08,;6.55,.23,;7.88,1,;5.22,1,;5.22,2.54,;3.88,.23,;2.55,1,;1.22,.23,;1.22,-1.31,;-.12,-2.08,;-1.45,-1.31,;-2.78,-2.08,;-2.78,-3.62,;-4.11,-1.32,;-4.11,.23,;-5.45,1,;-6.85,.37,;-7.25,-1.11,;-7.88,1.52,;-7.11,2.85,;-5.61,2.53,;-4.52,3.62,;-2.78,1,;-2.78,2.54,;-1.45,.23,;-.12,1,;-.12,2.54,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586153
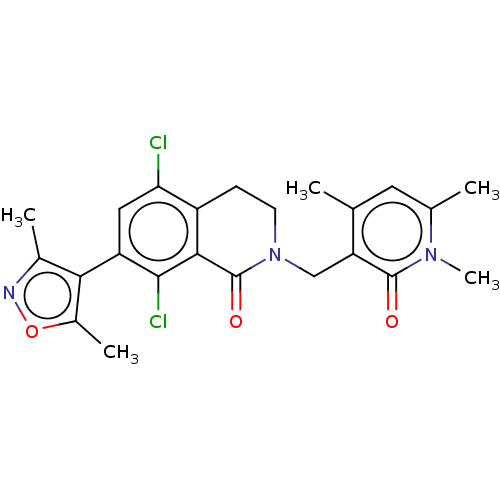
(CHEMBL5078476)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)n(C)c3=O)C(=O)c2c1Cl |(-7.25,-1.11,;-6.85,.37,;-7.88,1.52,;-7.11,2.85,;-5.61,2.53,;-4.52,3.62,;-5.45,1,;-4.11,.23,;-4.11,-1.32,;-2.78,-2.08,;-2.78,-3.62,;-1.45,-1.31,;-.12,-2.08,;1.22,-1.31,;1.22,.23,;2.55,1,;3.88,.23,;3.88,-1.31,;2.55,-2.08,;5.22,-2.08,;6.55,-1.31,;7.88,-2.08,;6.55,.23,;7.88,1,;5.22,1,;5.22,2.54,;-.12,1,;-.12,2.54,;-1.45,.23,;-2.78,1,;-2.78,2.54,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709

(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 in HEK293T cells assessed as H3K27me3 levels incubated for 48 hrs by flow cytometry |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586159

(CHEMBL5087917)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCNc2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.78,;2.93,-1.29,;4.02,-.2,;5.51,-.6,;6.6,.49,;8.09,.09,;6.2,1.98,;4.71,2.37,;4.31,3.86,;3.62,1.29,;2.14,1.68,;1.05,.6,;1.72,-.78,;1.05,-2.15,;-.45,-2.51,;-1.65,-1.55,;-2.98,-2.32,;-2.98,-3.86,;-4.32,-1.56,;-4.32,-.01,;-5.65,.76,;-7.06,.13,;-7.45,-1.36,;-8.09,1.27,;-7.32,2.61,;-5.81,2.29,;-4.72,3.38,;-2.98,.75,;-2.98,2.29,;-1.65,-.01,;-.46,.94,;-.85,2.42,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 in HEK293T cells assessed as H3K27me3 levels incubated for 48 hrs by flow cytometry |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586161

(CHEMBL5085040)Show SMILES CCN1CCN(Cc2c(OC)cc(C)[nH]c2=O)C(=O)c2c(Cl)c(cc(Cl)c12)-c1c(C)noc1C |(.24,-4.48,;-.85,-3.39,;-.45,-1.9,;1.05,-1.54,;1.72,-.17,;1.05,1.21,;2.14,2.3,;3.62,1.9,;4.02,.41,;2.93,-.68,;3.33,-2.17,;5.51,.01,;6.6,1.1,;8.09,.7,;6.2,2.59,;4.71,2.99,;4.31,4.48,;-.46,1.55,;-.85,3.04,;-1.65,.6,;-2.98,1.37,;-2.98,2.91,;-4.32,.6,;-4.32,-.95,;-2.98,-1.71,;-2.98,-3.25,;-1.65,-.94,;-5.65,1.37,;-7.06,.74,;-7.45,-.74,;-8.09,1.89,;-7.32,3.22,;-5.81,2.9,;-4.72,3.99,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586164
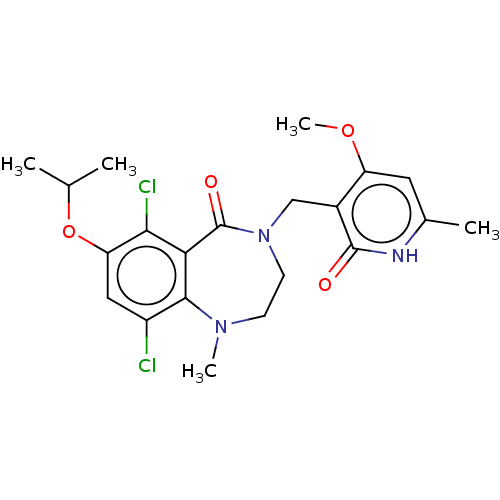
(CHEMBL5072578)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(OC(C)C)c(Cl)c2C1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586168

(CHEMBL5092735)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(c(Cl)c2C1=O)-c1ccc(cc1)N1CCOCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586169

(CHEMBL5069417)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(c(Cl)c2C1=O)-c1ccc(Cl)cc1F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586154

(CHEMBL5092557)Show SMILES CCc1cc(C)n(C)c(=O)c1CN1CCc2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(2.55,-3.62,;2.55,-2.08,;3.88,-1.31,;5.22,-2.08,;6.55,-1.31,;7.88,-2.08,;6.55,.23,;7.88,1,;5.22,1,;5.22,2.54,;3.88,.23,;2.55,1,;1.22,.23,;1.22,-1.31,;-.12,-2.08,;-1.45,-1.31,;-2.78,-2.08,;-2.78,-3.62,;-4.11,-1.32,;-4.11,.23,;-5.45,1,;-6.85,.37,;-7.25,-1.11,;-7.88,1.52,;-7.11,2.85,;-5.61,2.53,;-4.52,3.62,;-2.78,1,;-2.78,2.54,;-1.45,.23,;-.12,1,;-.12,2.54,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586160

(CHEMBL5085993)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.71,;2.93,-1.22,;4.02,-.13,;5.51,-.53,;6.6,.56,;8.09,.16,;6.2,2.05,;4.71,2.44,;4.31,3.93,;3.62,1.35,;2.14,1.75,;1.05,.66,;1.72,-.71,;1.05,-2.09,;-.45,-2.44,;-.85,-3.93,;-1.65,-1.48,;-2.98,-2.25,;-2.98,-3.79,;-4.32,-1.49,;-4.32,.05,;-5.65,.82,;-7.06,.2,;-7.45,-1.29,;-8.09,1.34,;-7.32,2.68,;-5.81,2.36,;-4.72,3.44,;-2.98,.82,;-2.98,2.36,;-1.65,.06,;-.46,1.01,;-.85,2.49,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 in HEK293T cells assessed as H3K27me3 levels incubated for 48 hrs by flow cytometry |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586170

(CHEMBL5079867)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCN(C)c2c(Cl)cc(c(Cl)c2C1=O)-c1ccc(CN2CCOCC2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586155

(CHEMBL5081796)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(cc(C)n(C)c3=O)C3CC3)C(=O)c2c1Cl |(-7.25,-1.11,;-6.85,.37,;-7.88,1.52,;-7.11,2.85,;-5.61,2.53,;-4.52,3.62,;-5.45,1,;-4.11,.23,;-4.11,-1.32,;-2.78,-2.08,;-2.78,-3.62,;-1.45,-1.31,;-.12,-2.08,;1.22,-1.31,;1.22,.23,;2.55,1,;3.88,.23,;3.88,-1.31,;5.22,-2.08,;6.55,-1.31,;7.88,-2.08,;6.55,.23,;7.88,1,;5.22,1,;5.22,2.54,;2.55,-2.08,;1.01,-2.08,;1.78,-3.41,;-.12,1,;-.12,2.54,;-1.45,.23,;-2.78,1,;-2.78,2.54,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 302 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586163

(CHEMBL5094306)Show SMILES CCN1CCN(Cc2c(OC)cc(C)[nH]c2=O)C(=O)c2c(Cl)c(ccc12)-c1c(C)noc1C |(.24,-4.48,;-.85,-3.39,;-.45,-1.9,;1.05,-1.54,;1.72,-.17,;1.05,1.21,;2.14,2.3,;3.62,1.9,;4.02,.41,;2.93,-.68,;3.33,-2.17,;5.51,.01,;6.6,1.1,;8.09,.7,;6.2,2.59,;4.71,2.99,;4.31,4.48,;-.46,1.55,;-.85,3.04,;-1.65,.6,;-2.98,1.37,;-2.98,2.91,;-4.32,.6,;-4.32,-.95,;-2.98,-1.71,;-1.65,-.94,;-5.65,1.37,;-7.06,.74,;-7.45,-.74,;-8.09,1.89,;-7.32,3.22,;-5.81,2.9,;-4.72,3.99,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 489 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586172
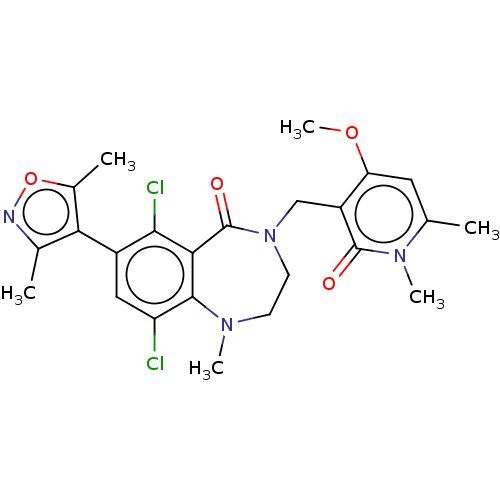
(CHEMBL5074685)Show SMILES COc1cc(C)n(C)c(=O)c1CN1CCN(C)c2c(Cl)cc(-c3c(C)noc3C)c(Cl)c2C1=O |(3.33,-2.71,;2.93,-1.22,;4.02,-.13,;5.51,-.53,;6.6,.56,;8.09,.16,;6.2,2.05,;7.29,3.13,;4.71,2.44,;4.31,3.93,;3.62,1.35,;2.14,1.75,;1.05,.66,;1.72,-.72,;1.05,-2.09,;-.45,-2.44,;-.85,-3.93,;-1.65,-1.48,;-2.98,-2.25,;-2.98,-3.79,;-4.32,-1.49,;-4.32,.05,;-5.65,.82,;-7.06,.2,;-7.45,-1.29,;-8.09,1.34,;-7.32,2.68,;-5.81,2.36,;-4.72,3.44,;-2.98,.82,;-2.98,2.36,;-1.65,.05,;-.46,1.01,;-.85,2.49,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 541 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586153

(CHEMBL5078476)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)n(C)c3=O)C(=O)c2c1Cl |(-7.25,-1.11,;-6.85,.37,;-7.88,1.52,;-7.11,2.85,;-5.61,2.53,;-4.52,3.62,;-5.45,1,;-4.11,.23,;-4.11,-1.32,;-2.78,-2.08,;-2.78,-3.62,;-1.45,-1.31,;-.12,-2.08,;1.22,-1.31,;1.22,.23,;2.55,1,;3.88,.23,;3.88,-1.31,;2.55,-2.08,;5.22,-2.08,;6.55,-1.31,;7.88,-2.08,;6.55,.23,;7.88,1,;5.22,1,;5.22,2.54,;-.12,1,;-.12,2.54,;-1.45,.23,;-2.78,1,;-2.78,2.54,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 in HEK293T cells assessed as H3K27me3 levels incubated for 48 hrs by flow cytometry |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50586156

(CHEMBL5093457)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(cc(C)n(C)c3=O)C(F)(F)F)C(=O)c2c1Cl |(-7.25,-1.11,;-6.85,.37,;-7.88,1.52,;-7.11,2.85,;-5.61,2.53,;-4.52,3.62,;-5.45,1,;-4.11,.23,;-4.11,-1.32,;-2.78,-2.08,;-2.78,-3.62,;-1.45,-1.31,;-.12,-2.08,;1.22,-1.31,;1.22,.23,;2.55,1,;3.88,.23,;3.88,-1.31,;5.22,-2.08,;6.55,-1.31,;7.88,-2.08,;6.55,.23,;7.88,1,;5.22,1,;5.22,2.54,;2.55,-2.08,;2.55,-3.62,;1.78,-.74,;1.01,-2.08,;-.12,1,;-.12,2.54,;-1.45,.23,;-2.78,1,;-2.78,2.54,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00448
BindingDB Entry DOI: 10.7270/Q2J10735 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data