 Found 19 hits with Last Name = 'kjaerulff' and Initial = 'l'
Found 19 hits with Last Name = 'kjaerulff' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50333649
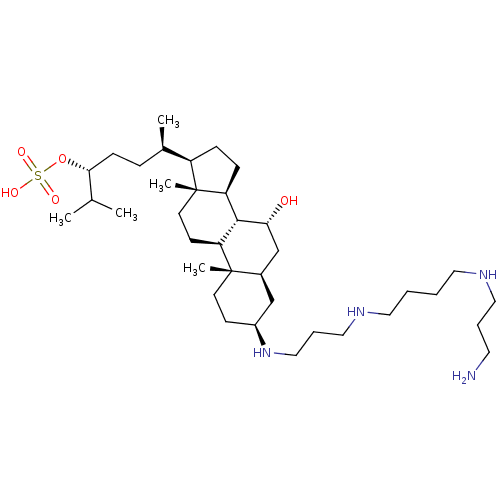
((3R,6R)-6-((3S,5R,7R,8R,9S,10S,13R,14S,17R)-3-(3-(...)Show SMILES CC(C)[C@@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)NCCCNCCCCNCCCN)OS(O)(=O)=O |r| Show InChI InChI=1S/C37H72N4O5S/c1-26(2)34(46-47(43,44)45)13-10-27(3)30-11-12-31-35-32(15-17-37(30,31)5)36(4)16-14-29(24-28(36)25-33(35)42)41-23-9-22-40-20-7-6-19-39-21-8-18-38/h26-35,39-42H,6-25,38H2,1-5H3,(H,43,44,45)/t27-,28-,29+,30-,31+,32+,33-,34-,35+,36+,37-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50209683
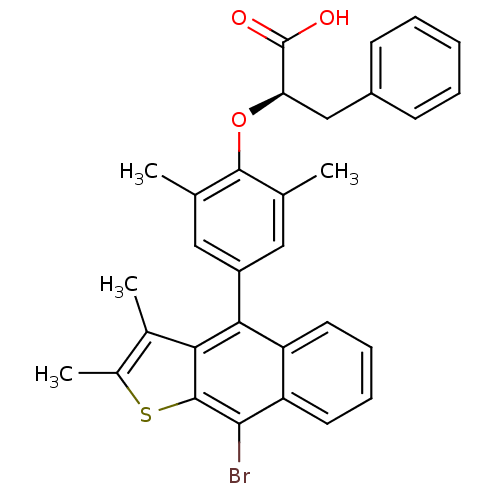
((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50575001
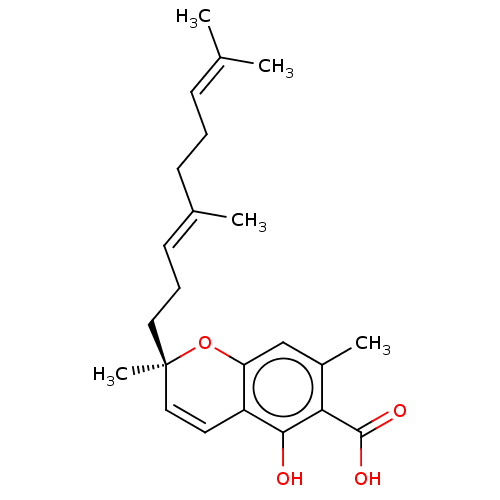
(CHEMBL2310311)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2cc(-[#6])c(-[#6](-[#8])=O)c(-[#8])c2-[#6]=[#6]1 |r,c:26| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50537951
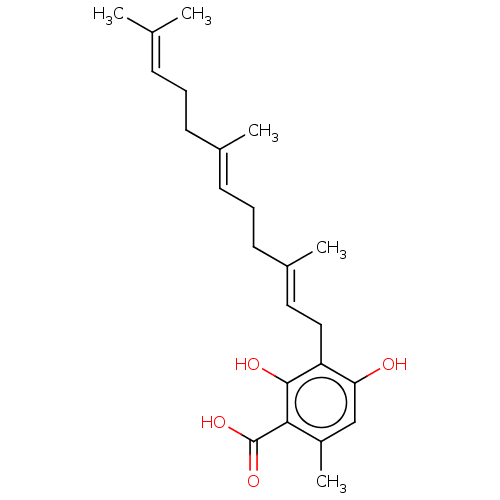
(Grifolic Acid)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(-[#6])c(-[#6](-[#8])=O)c1-[#8] Show InChI InChI=1S/C23H32O4/c1-15(2)8-6-9-16(3)10-7-11-17(4)12-13-19-20(24)14-18(5)21(22(19)25)23(26)27/h8,10,12,14,24-25H,6-7,9,11,13H2,1-5H3,(H,26,27)/b16-10+,17-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50574999
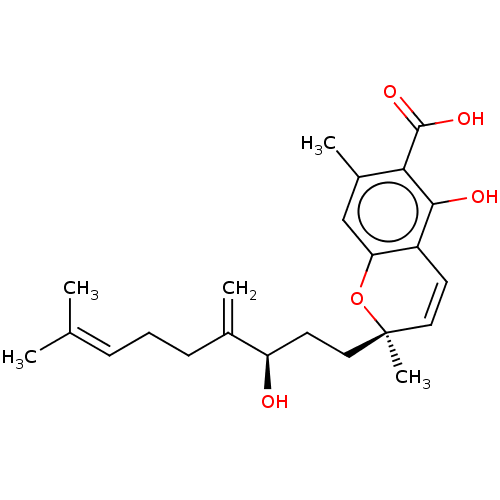
(CHEMBL4866095)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6](=[#6])-[#6@H](-[#8])-[#6]-[#6][C@@]1([#6])[#8]-c2cc(-[#6])c(-[#6](-[#8])=O)c(-[#8])c2-[#6]=[#6]1 |r,c:27| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50574998
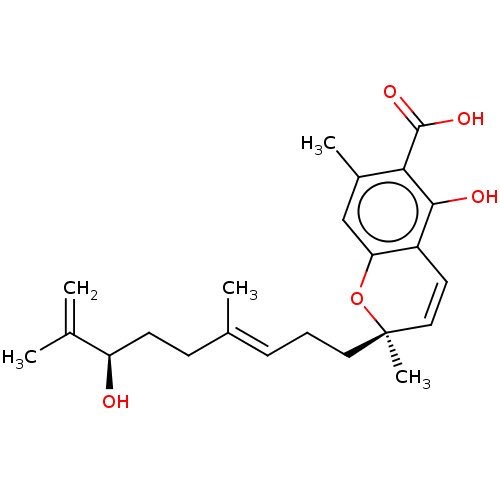
(CHEMBL4876070)Show SMILES CC(=C)[C@H](O)CC\C(C)=C\CC[C@@]1(C)Oc2cc(C)c(C(O)=O)c(O)c2C=C1 |r,c:27| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50614500

(CHEMBL233760)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(O)C(CO)OC1=O |c:17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50614498

(CHEMBL5276034)Show SMILES [#6]-[#6@@H](-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-c1cccc2c(-[#8])cc(-[#6])cc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50574997
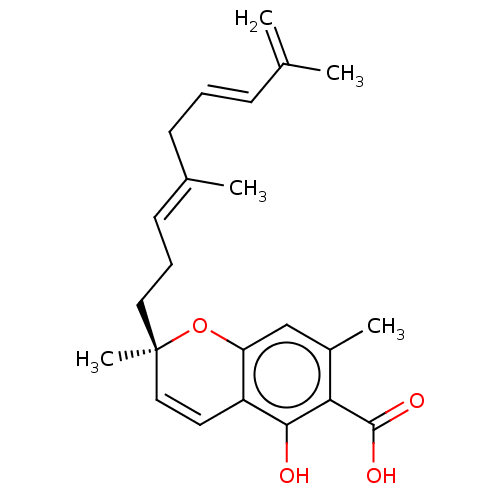
(CHEMBL4856719)Show SMILES CC(=C)\C=C\C\C(C)=C\CC[C@@]1(C)Oc2cc(C)c(C(O)=O)c(O)c2C=C1 |r,c:26| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50614495

(CHEMBL5290531)Show SMILES [H][C@]1([#6]-[#6]-[#6@@H](-[#6]-[#8])-c2c(-[#8])cc(-[#6])cc12)[#6@@H](-[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50574995
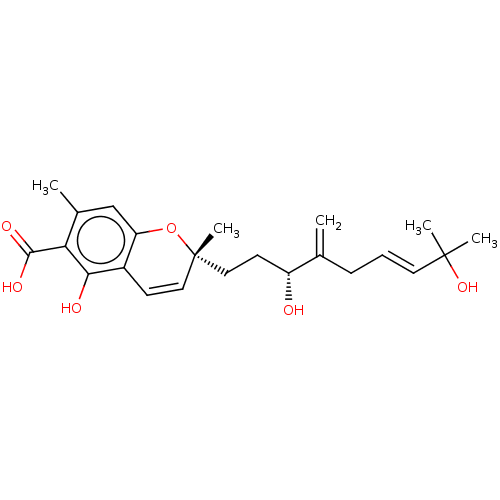
(CHEMBL4847372)Show SMILES Cc1cc2O[C@](C)(CC[C@@H](O)C(=C)C\C=C\C(C)(C)O)C=Cc2c(O)c1C(O)=O |r,c:20| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50614496

(CHEMBL5289765)Show SMILES [H][C@]1([#6]-[#6]-[#6@@H](-[#6]-[#8])-c2c(-[#8])cc(-[#6])cc12)[#6@@H](-[#6]-[#8])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50574996
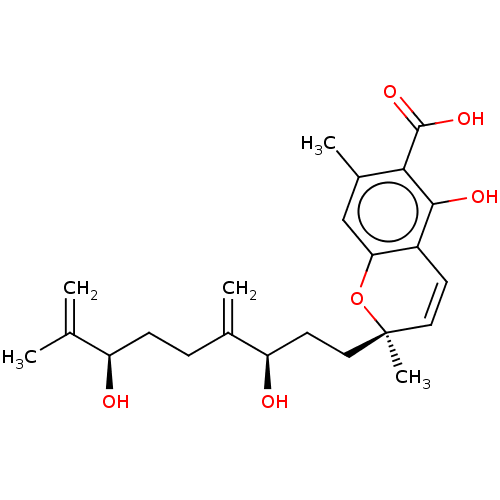
(CHEMBL4849561)Show SMILES CC(=C)[C@H](O)CCC(=C)[C@H](O)CC[C@@]1(C)Oc2cc(C)c(C(O)=O)c(O)c2C=C1 |r,c:28| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50575000
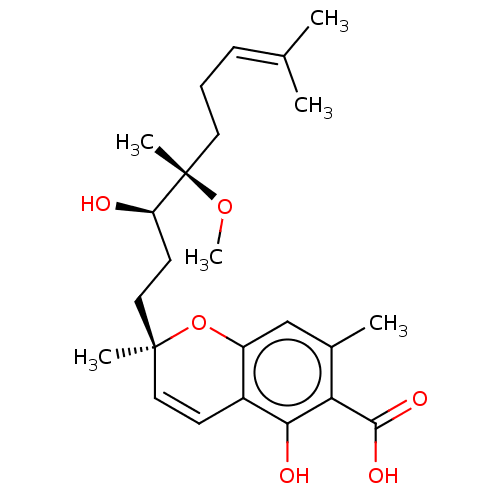
(CHEMBL4854129)Show SMILES [#6]-[#8][C@@]([#6])([#6]-[#6]\[#6]=[#6](\[#6])-[#6])[#6@H](-[#8])-[#6]-[#6][C@@]1([#6])[#8]-c2cc(-[#6])c(-[#6](-[#8])=O)c(-[#8])c2-[#6]=[#6]1 |r,c:29| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50614495

(CHEMBL5290531)Show SMILES [H][C@]1([#6]-[#6]-[#6@@H](-[#6]-[#8])-c2c(-[#8])cc(-[#6])cc12)[#6@@H](-[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50575002
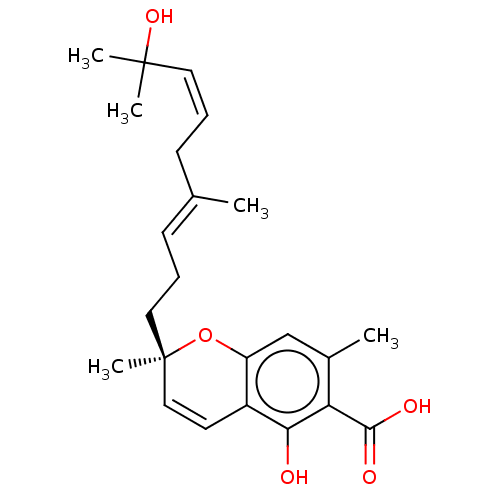
(CHEMBL4859368)Show SMILES C\C(C\C=C/C(C)(C)O)=C/CC[C@@]1(C)Oc2cc(C)c(C(O)=O)c(O)c2C=C1 |r,c:27| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00454
BindingDB Entry DOI: 10.7270/Q2BC43C6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50614497

(CHEMBL5281667)Show SMILES [H][C@]1([#6]-[#6@H](-[#8])-[#6@@H](-[#6])-c2c(-[#8])cc(-[#6])cc12)[#6@@H](-[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50614499

(CHEMBL5290371)Show SMILES [H][C@@]1([#6@@H](-[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])[#6@H](-[#8])-[#6]-[#6@@H](-[#6])-c2c(-[#8])cc(-[#6])cc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM23406

((3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5...)Show SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)OC(O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |t:37| Show InChI InChI=1S/C25H43NO18/c1-6-11(26-8-2-7(3-27)12(30)15(33)13(8)31)14(32)19(37)24(40-6)43-22-10(5-29)42-25(20(38)17(22)35)44-21-9(4-28)41-23(39)18(36)16(21)34/h2,6,8-39H,3-5H2,1H3/t6-,8+,9-,10-,11-,12-,13+,14+,15+,16-,17-,18-,19-,20-,21-,22-,23?,24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 5.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data