 Found 15773 hits with Last Name = 'qu' and Initial = 'l'
Found 15773 hits with Last Name = 'qu' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096105
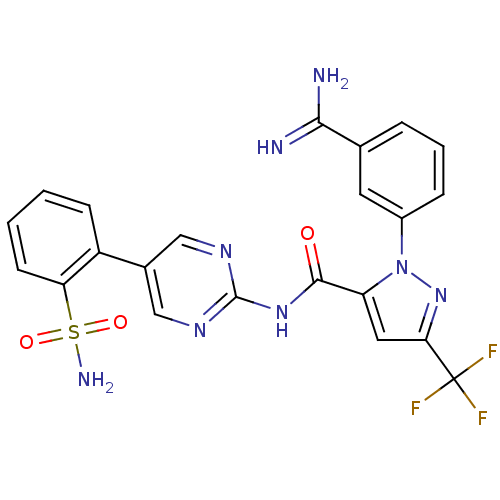
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ncc(cn1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C22H17F3N8O3S/c23-22(24,25)18-9-16(33(32-18)14-5-3-4-12(8-14)19(26)27)20(34)31-21-29-10-13(11-30-21)15-6-1-2-7-17(15)37(28,35)36/h1-11H,(H3,26,27)(H2,28,35,36)(H,29,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096099
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21FN6O3S/c1-14-11-21(31(30-14)17-6-4-5-16(12-17)23(26)27)24(32)29-20-10-9-15(13-19(20)25)18-7-2-3-8-22(18)35(28,33)34/h2-13H,1H3,(H3,26,27)(H,29,32)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096101
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cn2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C23H21N7O3S/c1-14-11-19(30(29-14)17-6-4-5-15(12-17)22(24)25)23(31)28-21-10-9-16(13-27-21)18-7-2-3-8-20(18)34(26,32)33/h2-13H,1H3,(H3,24,25)(H2,26,32,33)(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096110
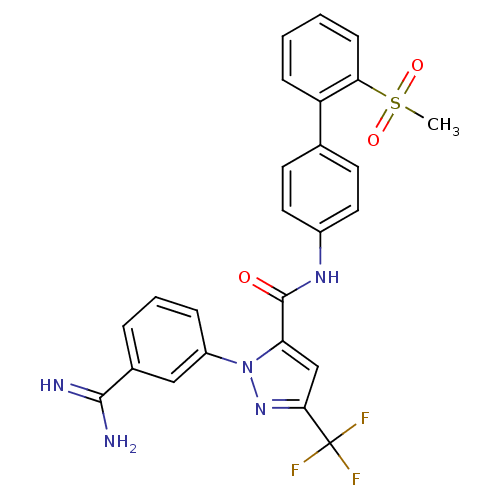
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(c2)C(N)=N)C(F)(F)F)cc1 Show InChI InChI=1S/C25H20F3N5O3S/c1-37(35,36)21-8-3-2-7-19(21)15-9-11-17(12-10-15)31-24(34)20-14-22(25(26,27)28)32-33(20)18-6-4-5-16(13-18)23(29)30/h2-14H,1H3,(H3,29,30)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096091
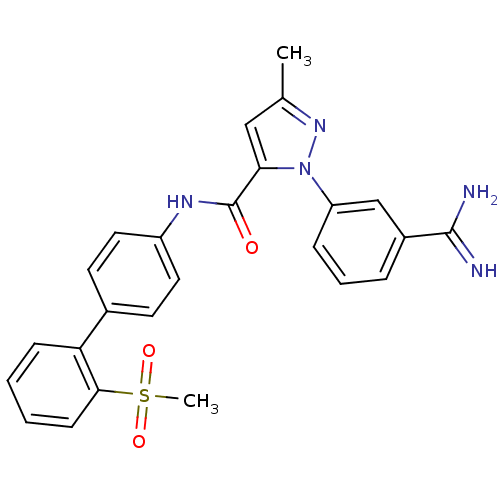
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(C)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C25H23N5O3S/c1-16-14-22(30(29-16)20-7-5-6-18(15-20)24(26)27)25(31)28-19-12-10-17(11-13-19)21-8-3-4-9-23(21)34(2,32)33/h3-15H,1-2H3,(H3,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096108
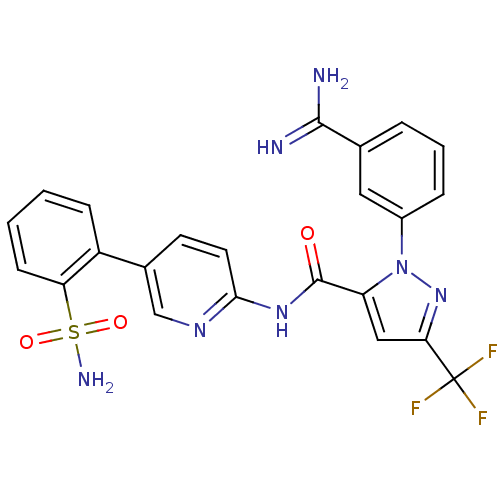
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ccc(cn1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C23H18F3N7O3S/c24-23(25,26)19-11-17(33(32-19)15-5-3-4-13(10-15)21(27)28)22(34)31-20-9-8-14(12-30-20)16-6-1-2-7-18(16)37(29,35)36/h1-12H,(H3,27,28)(H2,29,35,36)(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096085
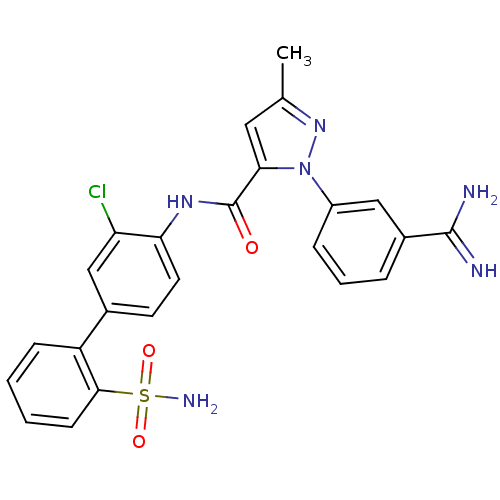
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2Cl)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21ClN6O3S/c1-14-11-21(31(30-14)17-6-4-5-16(12-17)23(26)27)24(32)29-20-10-9-15(13-19(20)25)18-7-2-3-8-22(18)35(28,33)34/h2-13H,1H3,(H3,26,27)(H,29,32)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50142700
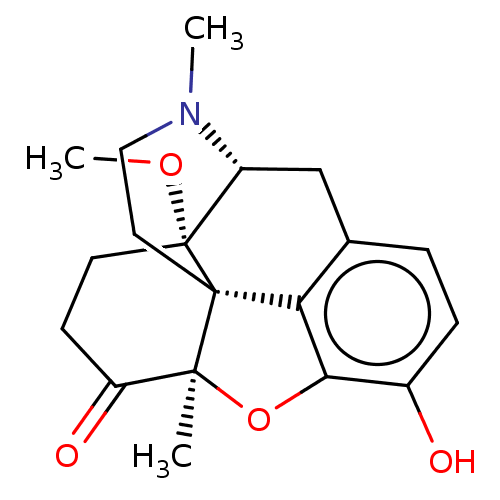
(CHEMBL326684)Show SMILES [H][C@@]12Cc3ccc(O)c4O[C@@]5(C)C(=O)CC[C@]1(OC)[C@@]5(CCN2C)c34 |r,TLB:23:22:16:2.3.24| Show InChI InChI=1S/C19H23NO4/c1-17-14(22)6-7-19(23-3)13-10-11-4-5-12(21)16(24-17)15(11)18(17,19)8-9-20(13)2/h4-5,13,21H,6-10H2,1-3H3/t13-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096098
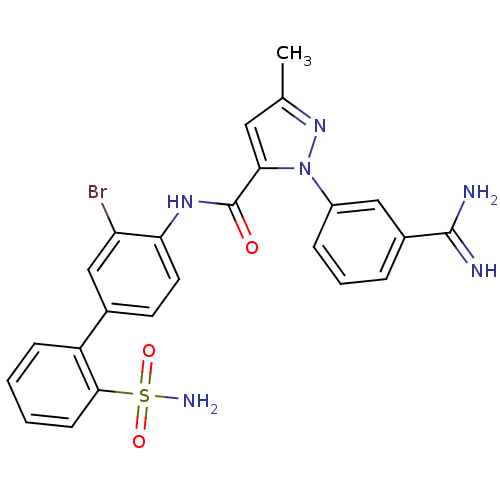
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2Br)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21BrN6O3S/c1-14-11-21(31(30-14)17-6-4-5-16(12-17)23(26)27)24(32)29-20-10-9-15(13-19(20)25)18-7-2-3-8-22(18)35(28,33)34/h2-13H,1H3,(H3,26,27)(H,29,32)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50528966
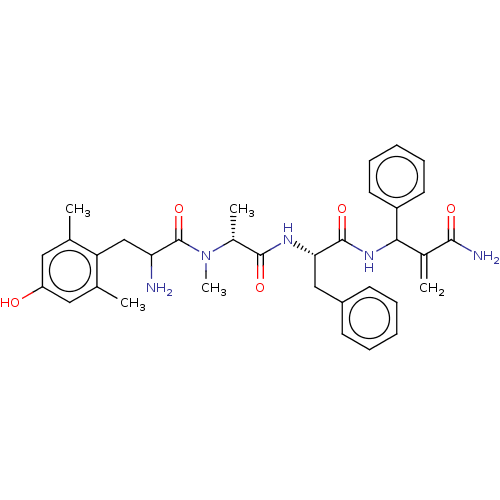
(CHEMBL4521879)Show SMILES C[C@@H](N(C)C(=O)C(N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC(C(=C)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C34H41N5O5/c1-20-16-26(40)17-21(2)27(20)19-28(35)34(44)39(5)23(4)32(42)37-29(18-24-12-8-6-9-13-24)33(43)38-30(22(3)31(36)41)25-14-10-7-11-15-25/h6-17,23,28-30,40H,3,18-19,35H2,1-2,4-5H3,(H2,36,41)(H,37,42)(H,38,43)/t23-,28?,29+,30?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation counting |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111701
BindingDB Entry DOI: 10.7270/Q2668HN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50528965
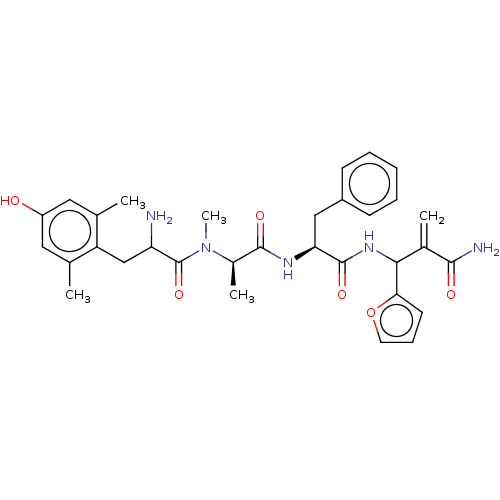
(CHEMBL4550234)Show SMILES C[C@@H](N(C)C(=O)C(N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC(C(=C)C(N)=O)c1ccco1 |r| Show InChI InChI=1S/C32H39N5O6/c1-18-14-23(38)15-19(2)24(18)17-25(33)32(42)37(5)21(4)30(40)35-26(16-22-10-7-6-8-11-22)31(41)36-28(20(3)29(34)39)27-12-9-13-43-27/h6-15,21,25-26,28,38H,3,16-17,33H2,1-2,4-5H3,(H2,34,39)(H,35,40)(H,36,41)/t21-,25?,26+,28?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation counting |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111701
BindingDB Entry DOI: 10.7270/Q2668HN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50528967
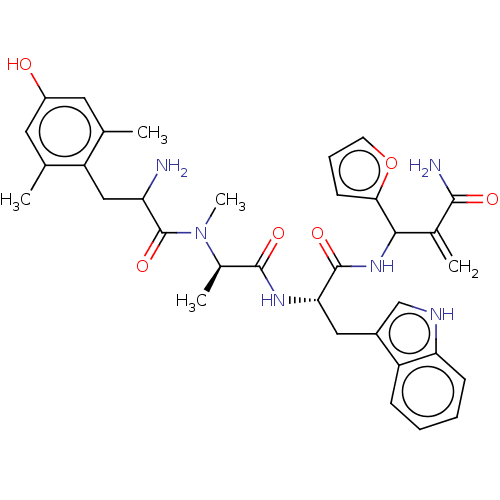
(CHEMBL4439415)Show SMILES C[C@@H](N(C)C(=O)C(N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC(C(=C)C(N)=O)c1ccco1 |r| Show InChI InChI=1S/C34H40N6O6/c1-18-13-23(41)14-19(2)25(18)16-26(35)34(45)40(5)21(4)32(43)38-28(15-22-17-37-27-10-7-6-9-24(22)27)33(44)39-30(20(3)31(36)42)29-11-8-12-46-29/h6-14,17,21,26,28,30,37,41H,3,15-16,35H2,1-2,4-5H3,(H2,36,42)(H,38,43)(H,39,44)/t21-,26?,28+,30?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation counting |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111701
BindingDB Entry DOI: 10.7270/Q2668HN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50528960
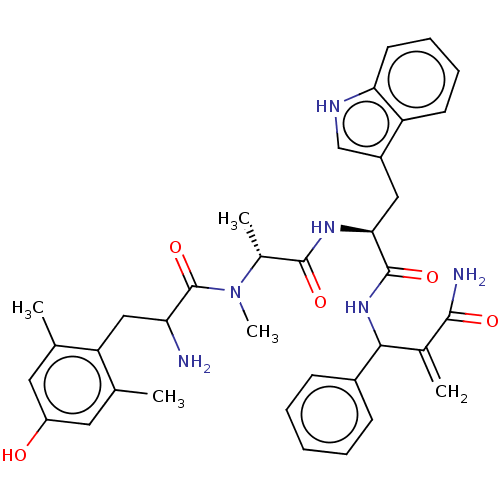
(CHEMBL4450250)Show SMILES C[C@@H](N(C)C(=O)C(N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC(C(=C)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C36H42N6O5/c1-20-15-26(43)16-21(2)28(20)18-29(37)36(47)42(5)23(4)34(45)40-31(17-25-19-39-30-14-10-9-13-27(25)30)35(46)41-32(22(3)33(38)44)24-11-7-6-8-12-24/h6-16,19,23,29,31-32,39,43H,3,17-18,37H2,1-2,4-5H3,(H2,38,44)(H,40,45)(H,41,46)/t23-,29?,31+,32?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation counting |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111701
BindingDB Entry DOI: 10.7270/Q2668HN9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374877
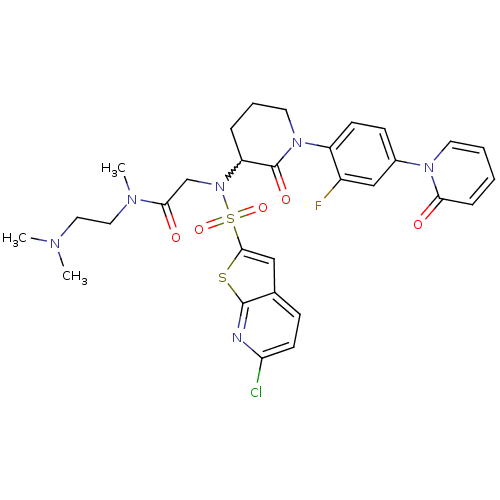
(CHEMBL270221)Show SMILES CN(C)CCN(C)C(=O)CN(C1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:11.10| Show InChI InChI=1S/C30H32ClFN6O5S2/c1-34(2)15-16-35(3)27(40)19-38(45(42,43)28-17-20-9-12-25(31)33-29(20)44-28)24-7-6-14-37(30(24)41)23-11-10-21(18-22(23)32)36-13-5-4-8-26(36)39/h4-5,8-13,17-18,24H,6-7,14-16,19H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096111
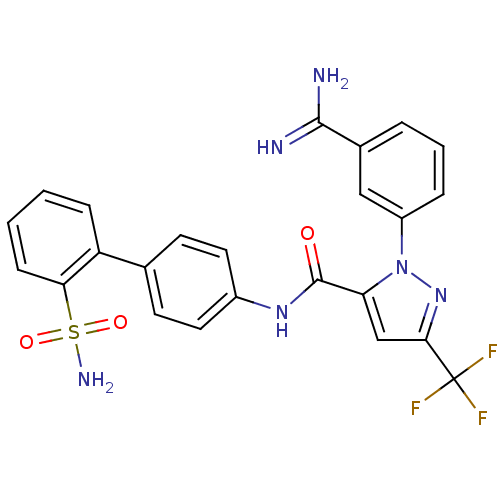
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3N6O3S/c25-24(26,27)21-13-19(33(32-21)17-5-3-4-15(12-17)22(28)29)23(34)31-16-10-8-14(9-11-16)18-6-1-2-7-20(18)37(30,35)36/h1-13H,(H3,28,29)(H,31,34)(H2,30,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50299217
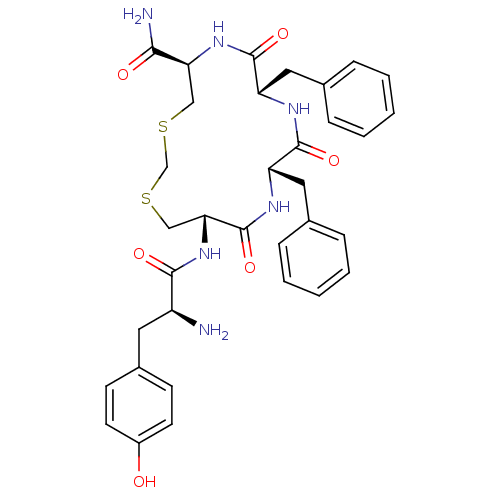
((5R,8S,11S,14S)-14-((S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C34H40N6O6S2/c35-25(15-23-11-13-24(41)14-12-23)31(43)40-29-19-48-20-47-18-28(30(36)42)39-33(45)27(17-22-9-5-2-6-10-22)37-32(44)26(38-34(29)46)16-21-7-3-1-4-8-21/h1-14,25-29,41H,15-20,35H2,(H2,36,42)(H,37,44)(H,38,46)(H,39,45)(H,40,43)/t25-,26-,27-,28-,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374879
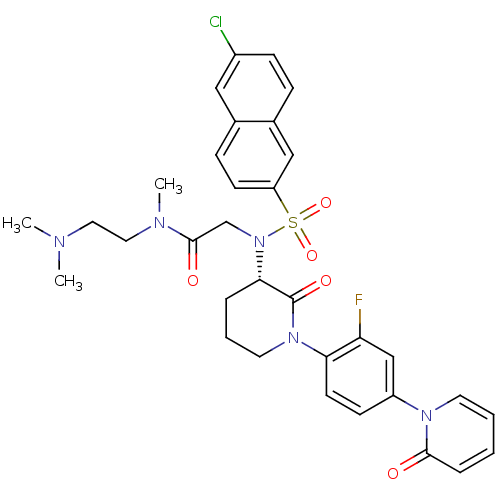
(CHEMBL401958)Show SMILES CN(C)CCN(C)C(=O)CN([C@H]1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C33H35ClFN5O5S/c1-36(2)17-18-37(3)32(42)22-40(46(44,45)27-13-10-23-19-25(34)11-9-24(23)20-27)30-7-6-16-39(33(30)43)29-14-12-26(21-28(29)35)38-15-5-4-8-31(38)41/h4-5,8-15,19-21,30H,6-7,16-18,22H2,1-3H3/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12852
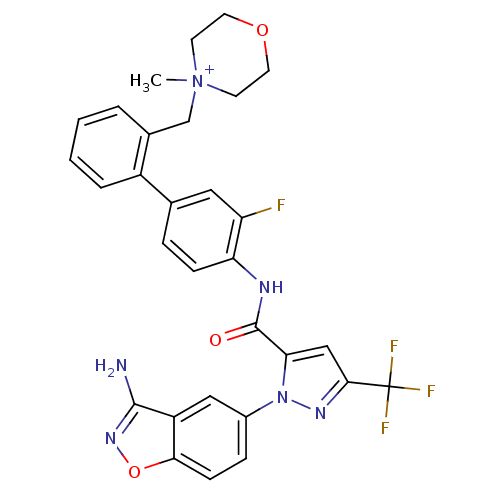
(4-{[2-(4-{[1-(3-amino-1,2-benzoxazol-5-yl)-3-(trif...)Show SMILES C[N+]1(Cc2ccccc2-c2ccc(NC(=O)c3cc(nn3-c3ccc4onc(N)c4c3)C(F)(F)F)c(F)c2)CCOCC1 Show InChI InChI=1S/C30H26F4N6O3/c1-40(10-12-42-13-11-40)17-19-4-2-3-5-21(19)18-6-8-24(23(31)14-18)36-29(41)25-16-27(30(32,33)34)37-39(25)20-7-9-26-22(15-20)28(35)38-43-26/h2-9,14-16H,10-13,17H2,1H3,(H2-,35,36,38,41)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | -60.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 1795-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.010
BindingDB Entry DOI: 10.7270/Q2FB515N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096096
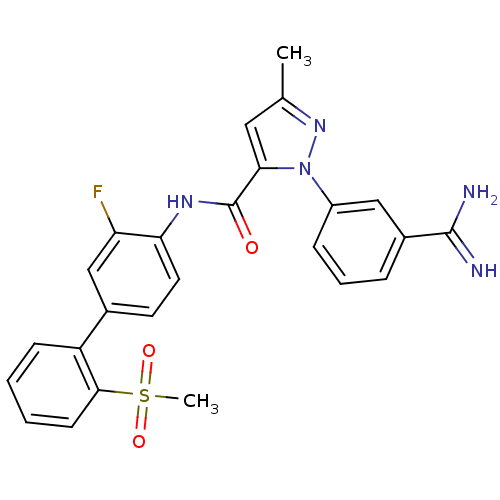
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)-c2ccccc2S(C)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C25H22FN5O3S/c1-15-12-22(31(30-15)18-7-5-6-17(13-18)24(27)28)25(32)29-21-11-10-16(14-20(21)26)19-8-3-4-9-23(19)35(2,33)34/h3-14H,1-2H3,(H3,27,28)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374879

(CHEMBL401958)Show SMILES CN(C)CCN(C)C(=O)CN([C@H]1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C33H35ClFN5O5S/c1-36(2)17-18-37(3)32(42)22-40(46(44,45)27-13-10-23-19-25(34)11-9-24(23)20-27)30-7-6-16-39(33(30)43)29-14-12-26(21-28(29)35)38-15-5-4-8-31(38)41/h4-5,8-15,19-21,30H,6-7,16-18,22H2,1-3H3/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374878
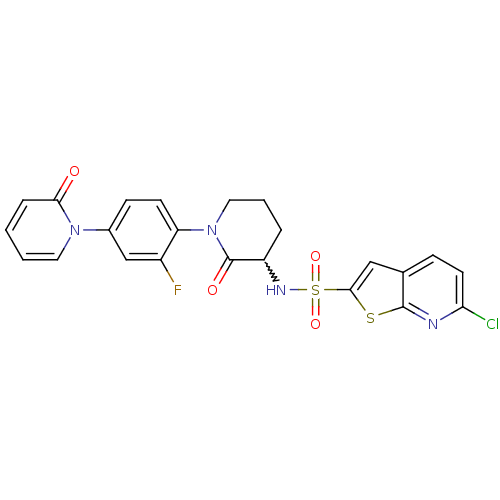
(CHEMBL270862)Show SMILES Fc1cc(ccc1N1CCCC(NS(=O)(=O)c2cc3ccc(Cl)nc3s2)C1=O)-n1ccccc1=O |w:11.12| Show InChI InChI=1S/C23H18ClFN4O4S2/c24-19-9-6-14-12-21(34-22(14)26-19)35(32,33)27-17-4-3-11-29(23(17)31)18-8-7-15(13-16(18)25)28-10-2-1-5-20(28)30/h1-2,5-10,12-13,17,27H,3-4,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50260646
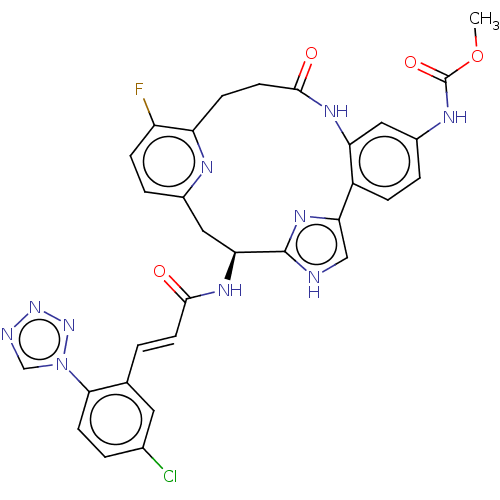
(CHEMBL4096251)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](Cc3ccc(F)c(CCC(=O)Nc2c1)n3)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C31H26ClFN10O4/c1-47-31(46)37-20-4-6-21-24(13-20)38-29(45)11-8-23-22(33)7-5-19(36-23)14-25(30-34-15-26(21)40-30)39-28(44)10-2-17-12-18(32)3-9-27(17)43-16-35-41-42-43/h2-7,9-10,12-13,15-16,25H,8,11,14H2,1H3,(H,34,40)(H,37,46)(H,38,45)(H,39,44)/b10-2+/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company, Research and Development, 350 Carter Road, Hopewell, NJ 08540 United States.
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using peptide substrate by spectrophotometry |
Bioorg Med Chem Lett 27: 4056-4060 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.048
BindingDB Entry DOI: 10.7270/Q2TB19B3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50142622
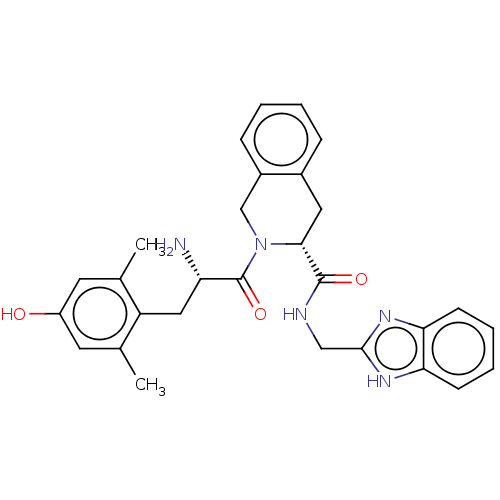
(CHEMBL3758292)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)NCc1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C29H31N5O3/c1-17-11-21(35)12-18(2)22(17)14-23(30)29(37)34-16-20-8-4-3-7-19(20)13-26(34)28(36)31-15-27-32-24-9-5-6-10-25(24)33-27/h3-12,23,26,35H,13-16,30H2,1-2H3,(H,31,36)(H,32,33)/t23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at delta opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097624
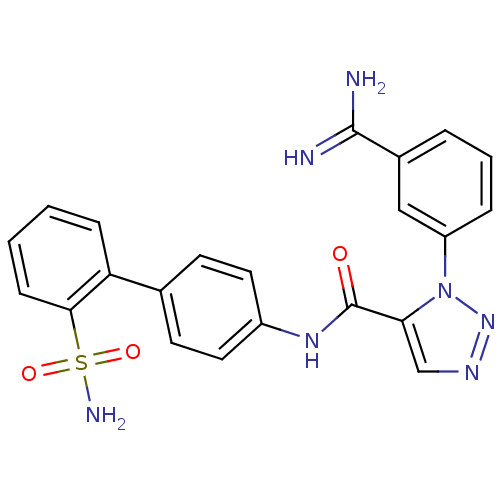
(3-(3-Carbamimidoyl-phenyl)-3H-[1,2,3]triazole-4-ca...)Show SMILES NC(=N)c1cccc(c1)-n1nncc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C22H19N7O3S/c23-21(24)15-4-3-5-17(12-15)29-19(13-26-28-29)22(30)27-16-10-8-14(9-11-16)18-6-1-2-7-20(18)33(25,31)32/h1-13H,(H3,23,24)(H,27,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089316
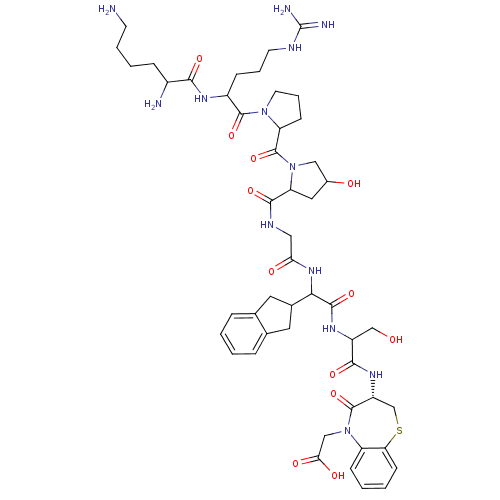
(CHEMBL410068 | H-Lys-Arg-Pro-Hyp-Gly-Igl-Ser-D-BT-...)Show SMILES NCCCCC(N)C(=O)NC(CCCNC(N)=N)C(=O)N1CCCC1C(=O)N1CC(O)CC1C(=O)NCC(=O)NC(C1Cc2ccccc2C1)C(=O)NC(CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C49H69N13O12S/c50-16-6-5-11-31(51)42(68)56-32(12-7-17-54-49(52)53)46(72)60-18-8-14-36(60)48(74)61-23-30(64)21-37(61)44(70)55-22-39(65)59-41(29-19-27-9-1-2-10-28(27)20-29)45(71)57-33(25-63)43(69)58-34-26-75-38-15-4-3-13-35(38)62(47(34)73)24-40(66)67/h1-4,9-10,13,15,29-34,36-37,41,63-64H,5-8,11-12,14,16-26,50-51H2,(H,55,70)(H,56,68)(H,57,71)(H,58,69)(H,59,65)(H,66,67)(H4,52,53,54)/t30?,31?,32?,33?,34-,36?,37?,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human cloned B1 receptor was determined using [3H]-[des-Arg10-Leu9]-kallidin as radioligand |
J Med Chem 43: 2387-94 (2000)
BindingDB Entry DOI: 10.7270/Q2N87913 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089314
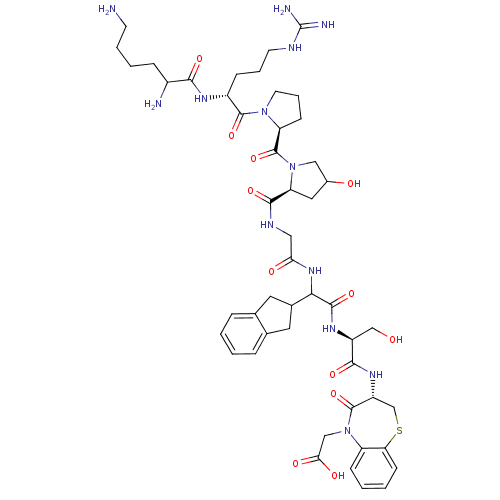
(CHEMBL405182 | H-Lys-Arg-Pro-Hyp-Gly-Igl-Ser-D-BT-...)Show SMILES NCCCCC(N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CC(O)C[C@H]1C(=O)NCC(=O)NC(C1Cc2ccccc2C1)C(=O)N[C@@H](CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C49H69N13O12S/c50-16-6-5-11-31(51)42(68)56-32(12-7-17-54-49(52)53)46(72)60-18-8-14-36(60)48(74)61-23-30(64)21-37(61)44(70)55-22-39(65)59-41(29-19-27-9-1-2-10-28(27)20-29)45(71)57-33(25-63)43(69)58-34-26-75-38-15-4-3-13-35(38)62(47(34)73)24-40(66)67/h1-4,9-10,13,15,29-34,36-37,41,63-64H,5-8,11-12,14,16-26,50-51H2,(H,55,70)(H,56,68)(H,57,71)(H,58,69)(H,59,65)(H,66,67)(H4,52,53,54)/t30?,31?,32-,33+,34-,36+,37+,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Ability to bind to human cloned B1 receptor in competition binding experiments with [3H][des-Arg10,Leu9]-Kallidin. |
J Med Chem 43: 2382-6 (2000)
BindingDB Entry DOI: 10.7270/Q2S181QZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50138568
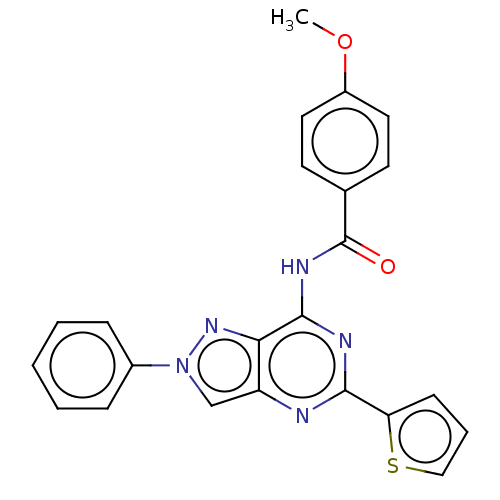
(CHEMBL3754229)Show SMILES COc1ccc(cc1)C(=O)Nc1nc(nc2cn(nc12)-c1ccccc1)-c1cccs1 Show InChI InChI=1S/C23H17N5O2S/c1-30-17-11-9-15(10-12-17)23(29)26-22-20-18(24-21(25-22)19-8-5-13-31-19)14-28(27-20)16-6-3-2-4-7-16/h2-14H,1H3,(H,24,25,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA at human A3A receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Eur J Med Chem 108: 117-33 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.015
BindingDB Entry DOI: 10.7270/Q2NK3GWZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human trypsin |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12693
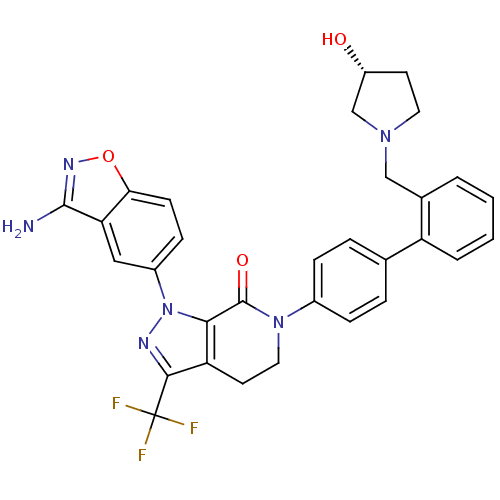
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H27F3N6O3/c32-31(33,34)28-24-12-14-39(30(42)27(24)40(36-28)21-9-10-26-25(15-21)29(35)37-43-26)20-7-5-18(6-8-20)23-4-2-1-3-19(23)16-38-13-11-22(41)17-38/h1-10,15,22,41H,11-14,16-17H2,(H2,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 4141-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.069
BindingDB Entry DOI: 10.7270/Q26T0JW1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12693

(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H27F3N6O3/c32-31(33,34)28-24-12-14-39(30(42)27(24)40(36-28)21-9-10-26-25(15-21)29(35)37-43-26)20-7-5-18(6-8-20)23-4-2-1-3-19(23)16-38-13-11-22(41)17-38/h1-10,15,22,41H,11-14,16-17H2,(H2,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5584-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.027
BindingDB Entry DOI: 10.7270/Q2Z899NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards Rabbit Coagulation factor X in a rabbit arterio-venous (A-V) shunt model |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230326
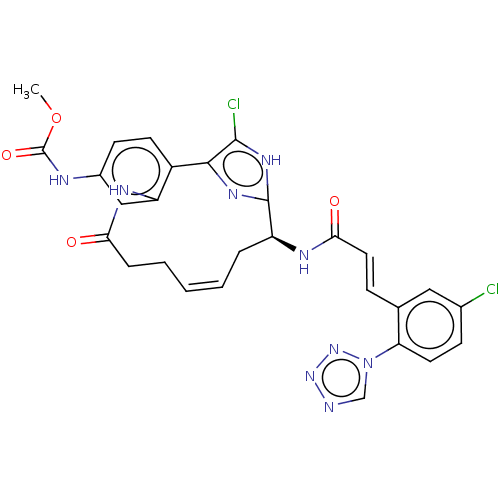
(CHEMBL4060950)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:18| Show InChI InChI=1S/C28H25Cl2N9O4/c1-43-28(42)32-18-9-10-19-21(14-18)34-23(40)6-4-2-3-5-20(27-35-25(19)26(30)36-27)33-24(41)12-7-16-13-17(29)8-11-22(16)39-15-31-37-38-39/h2-3,7-15,20H,4-6H2,1H3,(H,32,42)(H,33,41)(H,34,40)(H,35,36)/b3-2+,12-7+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro activity against rabbit FXa. |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230326

(CHEMBL4060950)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:18| Show InChI InChI=1S/C28H25Cl2N9O4/c1-43-28(42)32-18-9-10-19-21(14-18)34-23(40)6-4-2-3-5-20(27-35-25(19)26(30)36-27)33-24(41)12-7-16-13-17(29)8-11-22(16)39-15-31-37-38-39/h2-3,7-15,20H,4-6H2,1H3,(H,32,42)(H,33,41)(H,34,40)(H,35,36)/b3-2+,12-7+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method |
J Med Chem 60: 1060-1075 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01460
BindingDB Entry DOI: 10.7270/Q25D8V31 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50142621
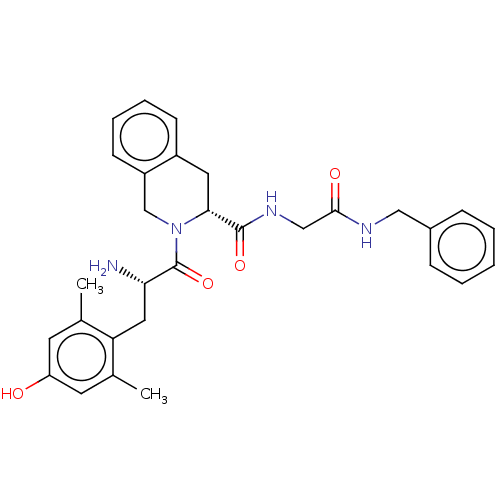
(CHEMBL3759292)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)NCC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C30H34N4O4/c1-19-12-24(35)13-20(2)25(19)15-26(31)30(38)34-18-23-11-7-6-10-22(23)14-27(34)29(37)33-17-28(36)32-16-21-8-4-3-5-9-21/h3-13,26-27,35H,14-18,31H2,1-2H3,(H,32,36)(H,33,37)/t26-,27+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089307

(CHEMBL410579 | H-Lys-Lys-Arg-Pro-Hyp-Gly-Igl-Ser-D...)Show SMILES NCCCCC(N)C(=O)NC(CCCCN)C(=O)N[C@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CC(O)C[C@H]1C(=O)NCC(=O)NC(C1Cc2ccccc2C1)C(=O)N[C@@H](CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C55H81N15O13S/c56-19-7-5-13-35(58)47(76)63-36(14-6-8-20-57)48(77)64-37(15-9-21-61-55(59)60)52(81)68-22-10-17-41(68)54(83)69-27-34(72)25-42(69)50(79)62-26-44(73)67-46(33-23-31-11-1-2-12-32(31)24-33)51(80)65-38(29-71)49(78)66-39-30-84-43-18-4-3-16-40(43)70(53(39)82)28-45(74)75/h1-4,11-12,16,18,33-39,41-42,46,71-72H,5-10,13-15,17,19-30,56-58H2,(H,62,79)(H,63,76)(H,64,77)(H,65,80)(H,66,78)(H,67,73)(H,74,75)(H4,59,60,61)/t34?,35?,36?,37-,38+,39-,41+,42+,46?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Ability to bind to human cloned B1 receptor in competition binding experiments with [3H][des-Arg10,Leu9]-Kallidin. |
J Med Chem 43: 2382-6 (2000)
BindingDB Entry DOI: 10.7270/Q2S181QZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374876
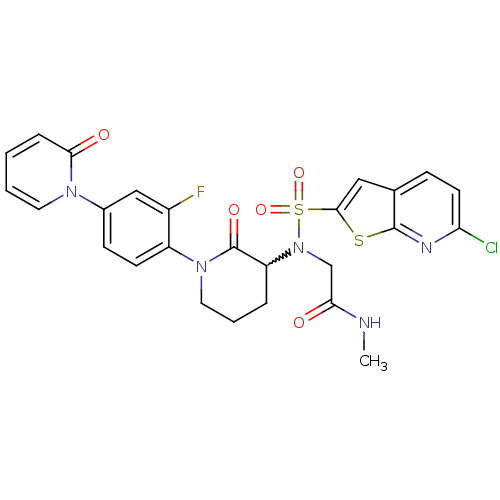
(CHEMBL270034)Show SMILES CNC(=O)CN(C1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:6.5| Show InChI InChI=1S/C26H23ClFN5O5S2/c1-29-22(34)15-33(40(37,38)24-13-16-7-10-21(27)30-25(16)39-24)20-5-4-12-32(26(20)36)19-9-8-17(14-18(19)28)31-11-3-2-6-23(31)35/h2-3,6-11,13-14,20H,4-5,12,15H2,1H3,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097626
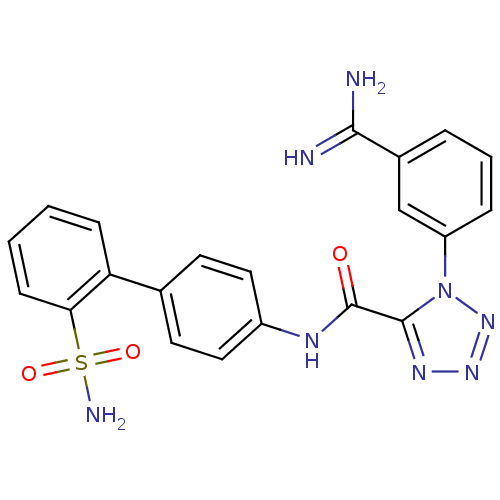
(1-(3-Carbamimidoyl-phenyl)-1H-tetrazole-5-carboxyl...)Show SMILES NC(=N)c1cccc(c1)-n1nnnc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C21H18N8O3S/c22-19(23)14-4-3-5-16(12-14)29-20(26-27-28-29)21(30)25-15-10-8-13(9-11-15)17-6-1-2-7-18(17)33(24,31)32/h1-12H,(H3,22,23)(H,25,30)(H2,24,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of human purified factor Xa |
Bioorg Med Chem Lett 13: 369-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BG2NC3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097626

(1-(3-Carbamimidoyl-phenyl)-1H-tetrazole-5-carboxyl...)Show SMILES NC(=N)c1cccc(c1)-n1nnnc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C21H18N8O3S/c22-19(23)14-4-3-5-16(12-14)29-20(26-27-28-29)21(30)25-15-10-8-13(9-11-15)17-6-1-2-7-18(17)33(24,31)32/h1-12H,(H3,22,23)(H,25,30)(H2,24,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50419921
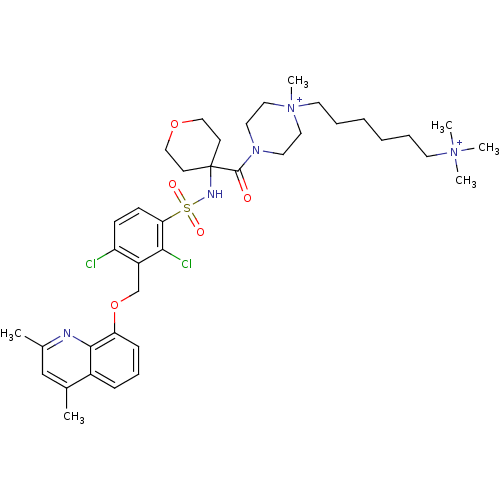
(CHEMBL1956864)Show SMILES Cc1cc(C)c2cccc(OCc3c(Cl)ccc(c3Cl)S(=O)(=O)NC3(CCOCC3)C(=O)N3CC[N+](C)(CCCCCC[N+](C)(C)C)CC3)c2n1 Show InChI InChI=1S/C38H55Cl2N5O5S/c1-28-26-29(2)41-36-30(28)12-11-13-33(36)50-27-31-32(39)14-15-34(35(31)40)51(47,48)42-38(16-24-49-25-17-38)37(46)43-18-22-45(6,23-19-43)21-10-8-7-9-20-44(3,4)5/h11-15,26,42H,7-10,16-25,27H2,1-6H3/q+2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting |
Bioorg Med Chem 20: 2091-100 (2012)
Article DOI: 10.1016/j.bmc.2012.01.036
BindingDB Entry DOI: 10.7270/Q2PN96WX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12681
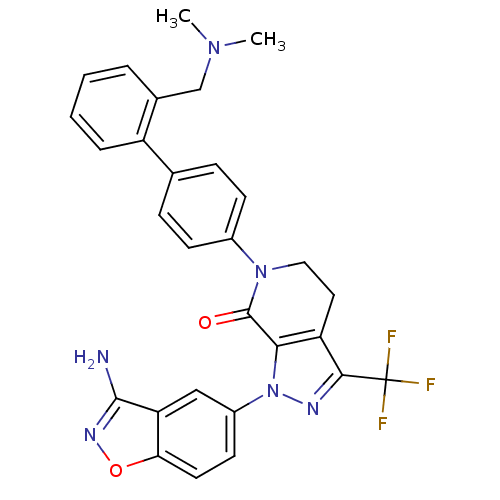
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)N1CCc2c(nn(c2C1=O)-c1ccc2onc(N)c2c1)C(F)(F)F Show InChI InChI=1S/C29H25F3N6O2/c1-36(2)16-18-5-3-4-6-21(18)17-7-9-19(10-8-17)37-14-13-22-25(28(37)39)38(34-26(22)29(30,31)32)20-11-12-24-23(15-20)27(33)35-40-24/h3-12,15H,13-14,16H2,1-2H3,(H2,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 4141-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.069
BindingDB Entry DOI: 10.7270/Q26T0JW1 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12681

(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)N1CCc2c(nn(c2C1=O)-c1ccc2onc(N)c2c1)C(F)(F)F Show InChI InChI=1S/C29H25F3N6O2/c1-36(2)16-18-5-3-4-6-21(18)17-7-9-19(10-8-17)37-14-13-22-25(28(37)39)38(34-26(22)29(30,31)32)20-11-12-24-23(15-20)27(33)35-40-24/h3-12,15H,13-14,16H2,1-2H3,(H2,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5584-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.027
BindingDB Entry DOI: 10.7270/Q2Z899NQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50096792
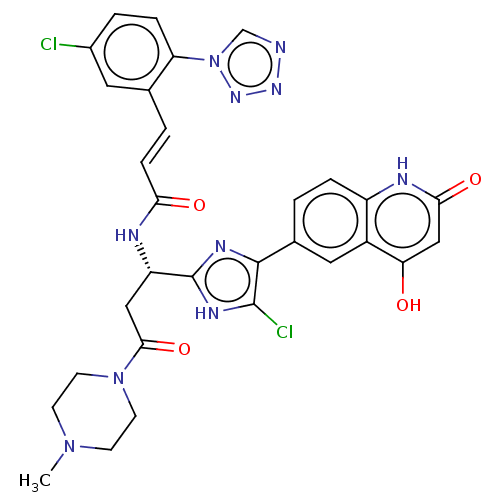
(CHEMBL3580759)Show SMILES CN1CCN(CC1)C(=O)C[C@H](NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1)c1nc(c(Cl)[nH]1)-c1ccc2[nH]c(=O)cc(O)c2c1 |r| Show InChI InChI=1S/C30H26Cl2N10O4/c1-40-8-10-41(11-9-40)27(46)14-22(35-25(44)7-3-17-12-19(31)4-6-23(17)42-16-33-38-39-42)30-36-28(29(32)37-30)18-2-5-21-20(13-18)24(43)15-26(45)34-21/h2-7,12-13,15-16,22,43H,8-11,14H2,1H3,(H,35,44)/b7-3+,28-18-/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometry |
ACS Med Chem Lett 6: 590-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00066
BindingDB Entry DOI: 10.7270/Q2B27X24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50369433
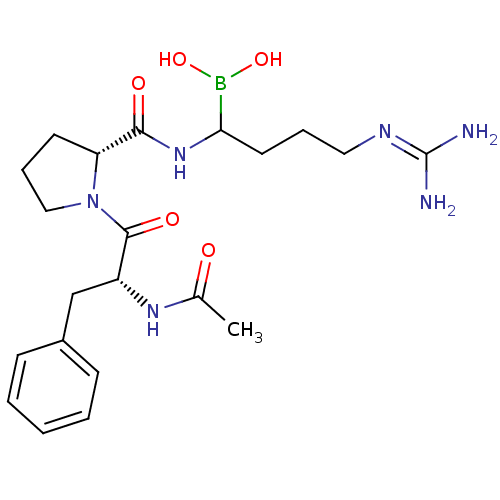
(CHEMBL1202108)Show SMILES [#6]-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#5](-[#8])-[#8] |r| Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to purified human coagulation factor Xa (FXa) |
J Med Chem 42: 2752-9 (1999)
Article DOI: 10.1021/jm980405i
BindingDB Entry DOI: 10.7270/Q28P616C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096094
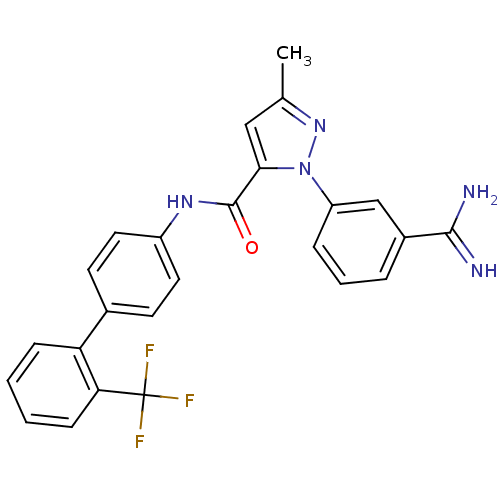
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2C(F)(F)F)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C25H20F3N5O/c1-15-13-22(33(32-15)19-6-4-5-17(14-19)23(29)30)24(34)31-18-11-9-16(10-12-18)20-7-2-3-8-21(20)25(26,27)28/h2-14H,1H3,(H3,29,30)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288406
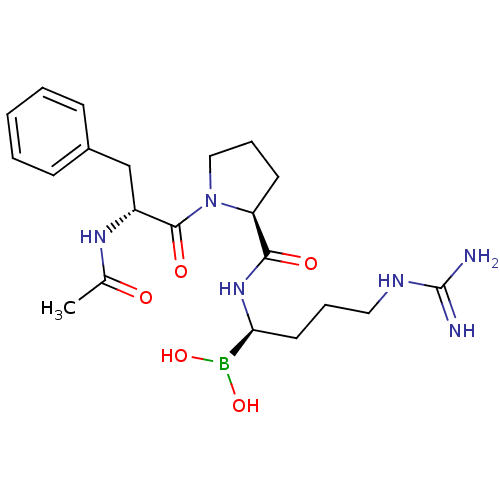
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096112
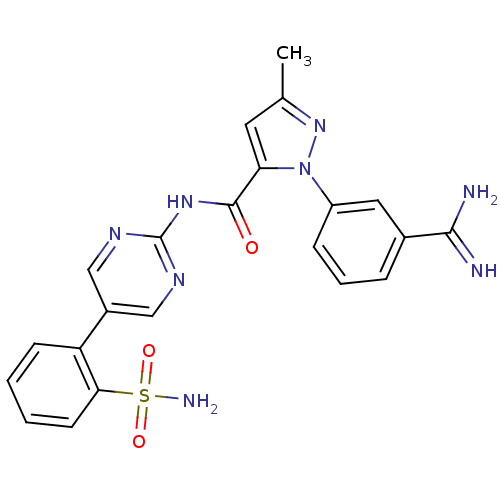
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ncc(cn2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C22H20N8O3S/c1-13-9-18(30(29-13)16-6-4-5-14(10-16)20(23)24)21(31)28-22-26-11-15(12-27-22)17-7-2-3-8-19(17)34(25,32)33/h2-12H,1H3,(H3,23,24)(H2,25,32,33)(H,26,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50142620
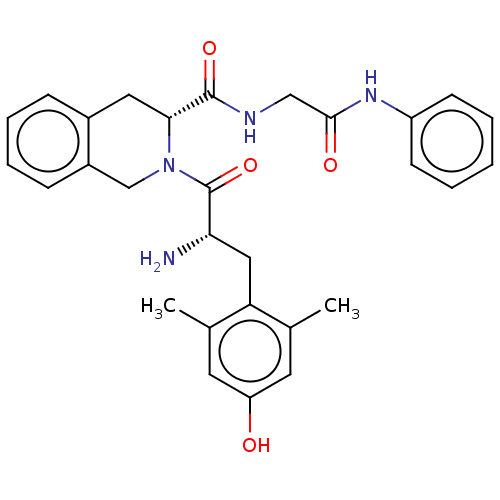
(CHEMBL3758712)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)NCC(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C29H32N4O4/c1-18-12-23(34)13-19(2)24(18)15-25(30)29(37)33-17-21-9-7-6-8-20(21)14-26(33)28(36)31-16-27(35)32-22-10-4-3-5-11-22/h3-13,25-26,34H,14-17,30H2,1-2H3,(H,31,36)(H,32,35)/t25-,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374871
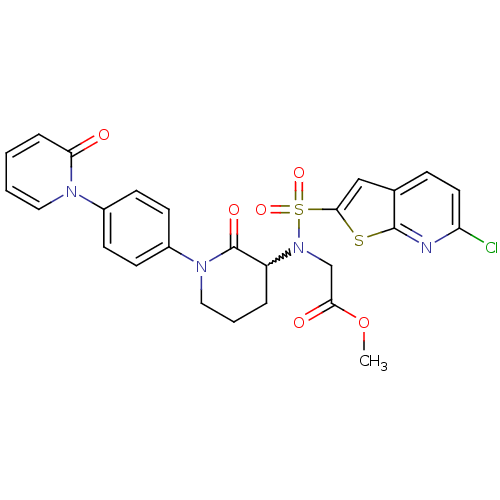
(CHEMBL258274)Show SMILES COC(=O)CN(C1CCCN(C1=O)c1ccc(cc1)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:6.5| Show InChI InChI=1S/C26H23ClN4O6S2/c1-37-23(33)16-31(39(35,36)24-15-17-7-12-21(27)28-25(17)38-24)20-5-4-14-30(26(20)34)19-10-8-18(9-11-19)29-13-3-2-6-22(29)32/h2-3,6-13,15,20H,4-5,14,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data