 Found 10 hits with Last Name = 'wessjohann' and Initial = 'l'
Found 10 hits with Last Name = 'wessjohann' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE by Ellman's method |
Bioorg Med Chem 18: 2173-7 (2010)
Article DOI: 10.1016/j.bmc.2010.01.074
BindingDB Entry DOI: 10.7270/Q2R78G5X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of bovine AChE by Elman's method |
Bioorg Med Chem 18: 2173-7 (2010)
Article DOI: 10.1016/j.bmc.2010.01.074
BindingDB Entry DOI: 10.7270/Q2R78G5X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE by Ellman's method |
Bioorg Med Chem 18: 2173-7 (2010)
Article DOI: 10.1016/j.bmc.2010.01.074
BindingDB Entry DOI: 10.7270/Q2R78G5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of bovine AChE by Elman's method |
Bioorg Med Chem 18: 2173-7 (2010)
Article DOI: 10.1016/j.bmc.2010.01.074
BindingDB Entry DOI: 10.7270/Q2R78G5X |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50222010
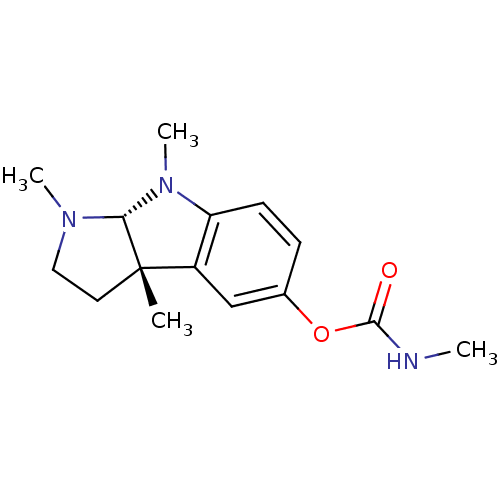
((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE at 100 uM by Ellman's method |
J Nat Prod 70: 1529-31 (2007)
Article DOI: 10.1021/np070259w
BindingDB Entry DOI: 10.7270/Q2GT5P1V |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of bovine AChE by Elman's method |
Bioorg Med Chem 18: 2173-7 (2010)
Article DOI: 10.1016/j.bmc.2010.01.074
BindingDB Entry DOI: 10.7270/Q2R78G5X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50308337
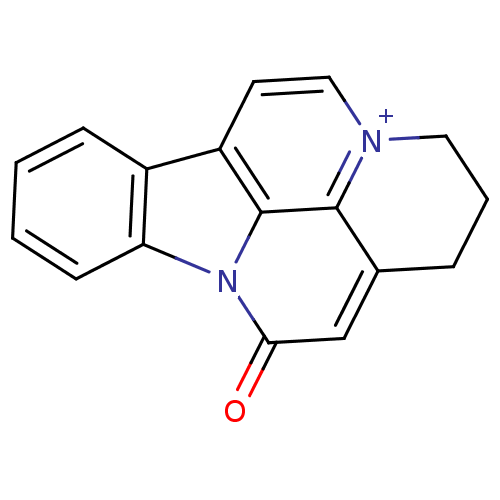
(CHEMBL589070 | Infractopicrin)Show InChI InChI=1S/C17H13N2O/c20-15-10-11-4-3-8-18-9-7-13-12-5-1-2-6-14(12)19(15)17(13)16(11)18/h1-2,5-7,9-10H,3-4,8H2/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of bovine AChE by Elman's method |
Bioorg Med Chem 18: 2173-7 (2010)
Article DOI: 10.1016/j.bmc.2010.01.074
BindingDB Entry DOI: 10.7270/Q2R78G5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50308338
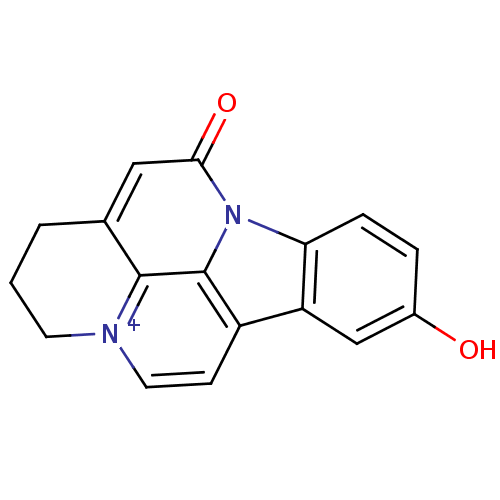
(10-Hydroxy-infractopicrin | CHEMBL589071)Show InChI InChI=1S/C17H12N2O2/c20-11-3-4-14-13(9-11)12-5-7-18-6-1-2-10-8-15(21)19(14)17(12)16(10)18/h3-5,7-9H,1-2,6H2/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of bovine AChE by Elman's method |
Bioorg Med Chem 18: 2173-7 (2010)
Article DOI: 10.1016/j.bmc.2010.01.074
BindingDB Entry DOI: 10.7270/Q2R78G5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE by Ellman's method |
Bioorg Med Chem 18: 2173-7 (2010)
Article DOI: 10.1016/j.bmc.2010.01.074
BindingDB Entry DOI: 10.7270/Q2R78G5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50222010

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of bovine AChE at 100 uM by Ellman's method |
J Nat Prod 70: 1529-31 (2007)
Article DOI: 10.1021/np070259w
BindingDB Entry DOI: 10.7270/Q2GT5P1V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data