 Found 336 hits with Last Name = 'adams' and Initial = 'la'
Found 336 hits with Last Name = 'adams' and Initial = 'la' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178953
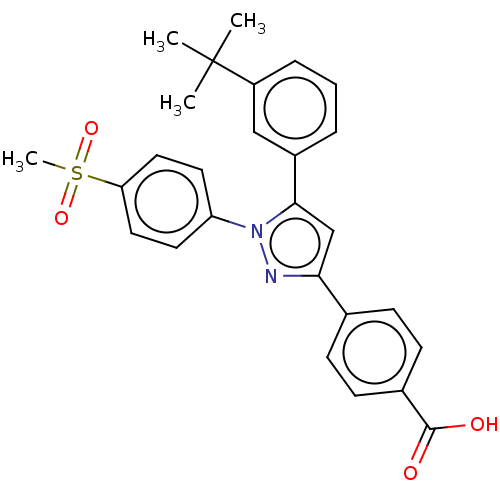
(CHEMBL3815166)Show SMILES CC(C)(C)c1cccc(c1)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C27H26N2O4S/c1-27(2,3)21-7-5-6-20(16-21)25-17-24(18-8-10-19(11-9-18)26(30)31)28-29(25)22-12-14-23(15-13-22)34(4,32)33/h5-17H,1-4H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178952

(CHEMBL3814574)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C31H32N2O4S/c1-30(2)16-17-31(3,4)26-18-22(10-15-25(26)30)28-19-27(20-6-8-21(9-7-20)29(34)35)32-33(28)23-11-13-24(14-12-23)38(5,36)37/h6-15,18-19H,16-17H2,1-5H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50200369
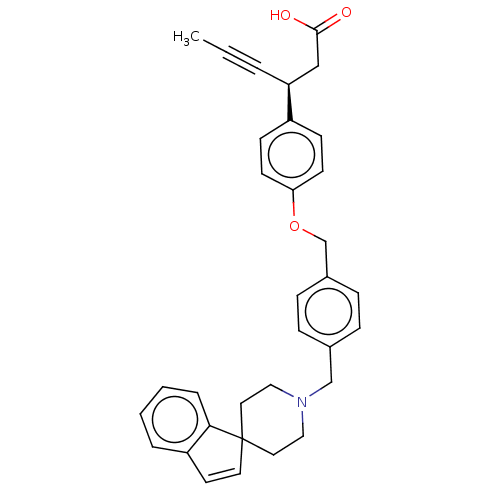
(CHEMBL3915620)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc(CN3CCC4(CC3)C=Cc3ccccc43)cc2)cc1 |r,c:26| Show InChI InChI=1S/C33H33NO3/c1-2-5-29(22-32(35)36)27-12-14-30(15-13-27)37-24-26-10-8-25(9-11-26)23-34-20-18-33(19-21-34)17-16-28-6-3-4-7-31(28)33/h3-4,6-17,29H,18-24H2,1H3,(H,35,36)/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178954

(CHEMBL3813779)Show SMILES Cc1cc(cc(c1)C(C)(C)C)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C28H28N2O4S/c1-18-14-21(16-22(15-18)28(2,3)4)26-17-25(19-6-8-20(9-7-19)27(31)32)29-30(26)23-10-12-24(13-11-23)35(5,33)34/h6-17H,1-5H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178961
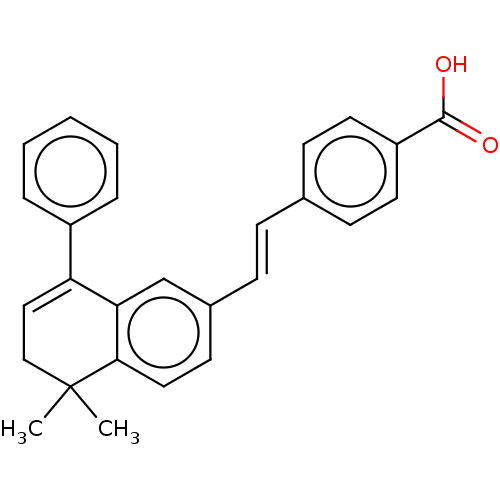
(CHEMBL2385268)Show SMILES CC1(C)CC=C(c2ccccc2)c2cc(\C=C\c3ccc(cc3)C(O)=O)ccc12 |t:4| Show InChI InChI=1S/C27H24O2/c1-27(2)17-16-23(21-6-4-3-5-7-21)24-18-20(12-15-25(24)27)9-8-19-10-13-22(14-11-19)26(28)29/h3-16,18H,17H2,1-2H3,(H,28,29)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50247164
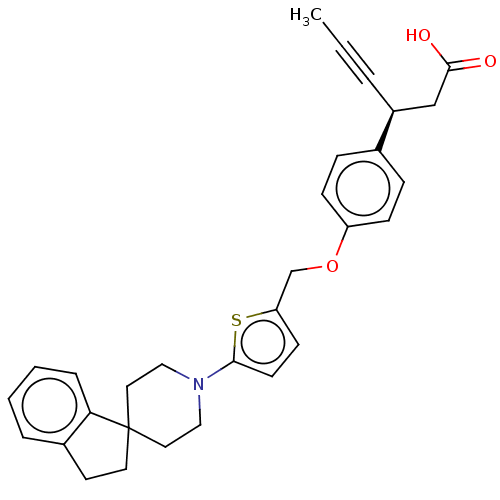
(CHEMBL4101901)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc(s2)N2CCC3(CCc4ccccc34)CC2)cc1 |r| Show InChI InChI=1S/C30H31NO3S/c1-2-5-24(20-29(32)33)22-8-10-25(11-9-22)34-21-26-12-13-28(35-26)31-18-16-30(17-19-31)15-14-23-6-3-4-7-27(23)30/h3-4,6-13,24H,14-21H2,1H3,(H,32,33)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50204020
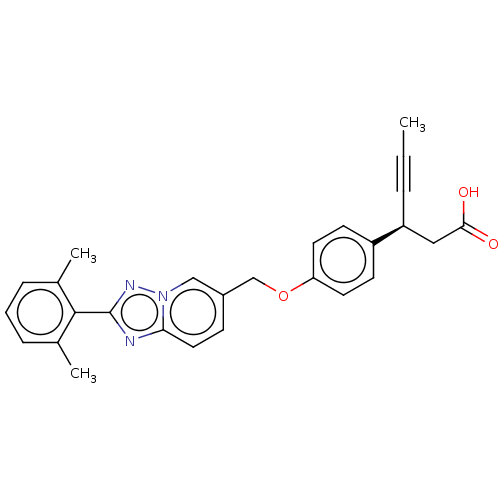
(CHEMBL3927519)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc3nc(nn3c2)-c2c(C)cccc2C)cc1 |r| Show InChI InChI=1S/C27H25N3O3/c1-4-6-22(15-25(31)32)21-10-12-23(13-11-21)33-17-20-9-14-24-28-27(29-30(24)16-20)26-18(2)7-5-8-19(26)3/h5,7-14,16,22H,15,17H2,1-3H3,(H,31,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50204012
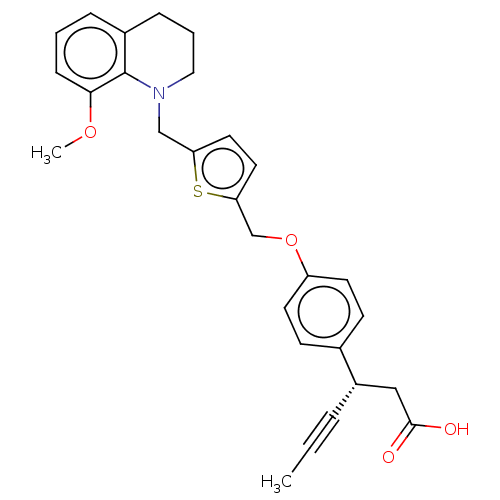
(CHEMBL3955132)Show SMILES COc1cccc2CCCN(Cc3ccc(COc4ccc(cc4)[C@H](CC(O)=O)C#CC)s3)c12 |r| Show InChI InChI=1S/C28H29NO4S/c1-3-6-22(17-27(30)31)20-10-12-23(13-11-20)33-19-25-15-14-24(34-25)18-29-16-5-8-21-7-4-9-26(32-2)28(21)29/h4,7,9-15,22H,5,8,16-19H2,1-2H3,(H,30,31)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50247166

(CHEMBL4082711)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc3nc(nn3c2)-c2ccc(cc2)C(C)C)cc1 |r| Show InChI InChI=1S/C28H27N3O3/c1-4-5-24(16-27(32)33)22-11-13-25(14-12-22)34-18-20-6-15-26-29-28(30-31(26)17-20)23-9-7-21(8-10-23)19(2)3/h6-15,17,19,24H,16,18H2,1-3H3,(H,32,33)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50247163
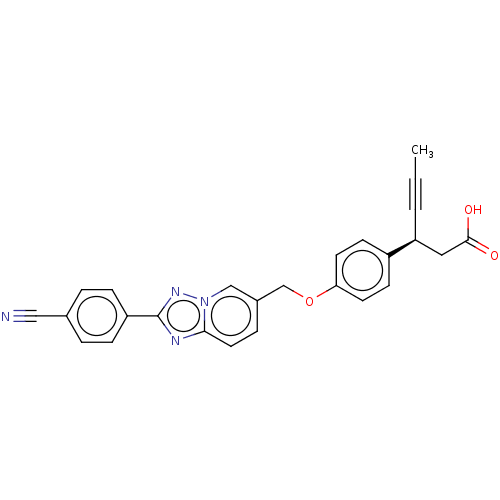
(CHEMBL4059614)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc3nc(nn3c2)-c2ccc(cc2)C#N)cc1 |r| Show InChI InChI=1S/C26H20N4O3/c1-2-3-22(14-25(31)32)20-9-11-23(12-10-20)33-17-19-6-13-24-28-26(29-30(24)16-19)21-7-4-18(15-27)5-8-21/h4-13,16,22H,14,17H2,1H3,(H,31,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50247156
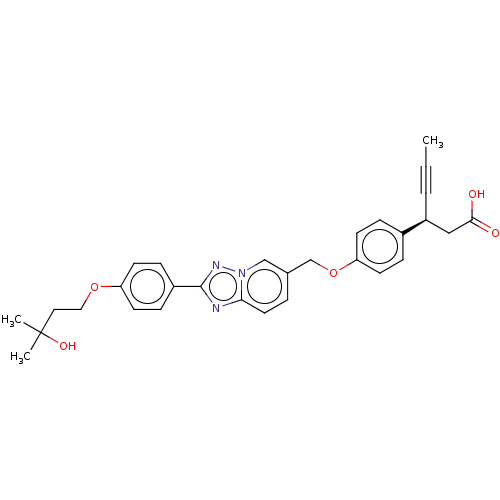
(CHEMBL4101472)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc3nc(nn3c2)-c2ccc(OCCC(C)(C)O)cc2)cc1 |r| Show InChI InChI=1S/C30H31N3O5/c1-4-5-24(18-28(34)35)22-7-11-26(12-8-22)38-20-21-6-15-27-31-29(32-33(27)19-21)23-9-13-25(14-10-23)37-17-16-30(2,3)36/h6-15,19,24,36H,16-18,20H2,1-3H3,(H,34,35)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50247167

(CHEMBL4097527)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc3nc(nn3c2)-c2ccc(OC(F)F)cc2)cc1 |r| Show InChI InChI=1S/C26H21F2N3O4/c1-2-3-20(14-24(32)33)18-5-9-21(10-6-18)34-16-17-4-13-23-29-25(30-31(23)15-17)19-7-11-22(12-8-19)35-26(27)28/h4-13,15,20,26H,14,16H2,1H3,(H,32,33)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50247162
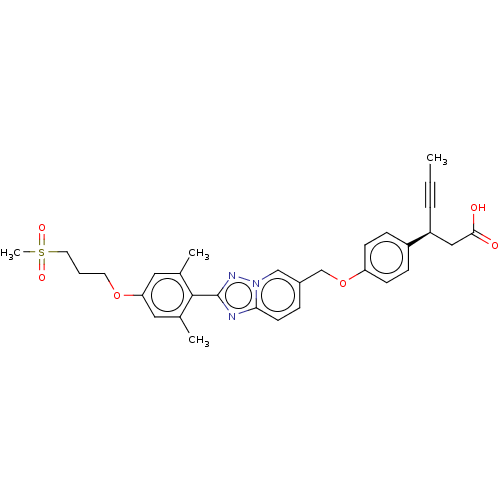
(CHEMBL4075819)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc3nc(nn3c2)-c2c(C)cc(OCCCS(C)(=O)=O)cc2C)cc1 |r| Show InChI InChI=1S/C31H33N3O6S/c1-5-7-25(18-29(35)36)24-9-11-26(12-10-24)40-20-23-8-13-28-32-31(33-34(28)19-23)30-21(2)16-27(17-22(30)3)39-14-6-15-41(4,37)38/h8-13,16-17,19,25H,6,14-15,18,20H2,1-4H3,(H,35,36)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178955

(CHEMBL3813965)Show SMILES CC(C)c1cc(cc(c1)C(C)(C)C)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C30H32N2O4S/c1-19(2)22-15-23(17-24(16-22)30(3,4)5)28-18-27(20-7-9-21(10-8-20)29(33)34)31-32(28)25-11-13-26(14-12-25)37(6,35)36/h7-19H,1-6H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50247165

(CHEMBL4077366)Show SMILES COCCOc1ccc(cc1)-c1nc2ccc(COc3ccc(cc3)[C@H](CC(O)=O)C#CC)cn2n1 |r| Show InChI InChI=1S/C28H27N3O5/c1-3-4-23(17-27(32)33)21-6-10-25(11-7-21)36-19-20-5-14-26-29-28(30-31(26)18-20)22-8-12-24(13-9-22)35-16-15-34-2/h5-14,18,23H,15-17,19H2,1-2H3,(H,32,33)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... |
J Med Chem 61: 934-945 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01411
BindingDB Entry DOI: 10.7270/Q2CZ39K5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178956

(CHEMBL3813807)Show SMILES CC(C)(C)c1cc(cc(c1)C(C)(C)C)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C31H34N2O4S/c1-30(2,3)23-16-22(17-24(18-23)31(4,5)6)28-19-27(20-8-10-21(11-9-20)29(34)35)32-33(28)25-12-14-26(15-13-25)38(7,36)37/h8-19H,1-7H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 461 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178957

(CHEMBL3814015)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1cc(cc(c1)C(C)(C)C)C(C)(C)C)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C32H37N3O4S/c1-31(2,3)24-17-23(18-25(19-24)32(4,5)6)29-20-28(21-9-11-22(12-10-21)30(36)37)33-35(29)26-13-15-27(16-14-26)40(38,39)34(7)8/h9-20H,1-8H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178962

(CHEMBL3814815)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)-n1nc(cc1-c1cc(cc(c1)C(C)(C)C)C(C)(C)C)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C36H42N4O3/c1-35(2,3)28-20-27(21-29(22-28)36(4,5)6)32-23-31(24-8-10-26(11-9-24)34(42)43)37-40(32)30-14-12-25(13-15-30)33(41)39-18-16-38(7)17-19-39/h8-15,20-23H,16-19H2,1-7H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178959
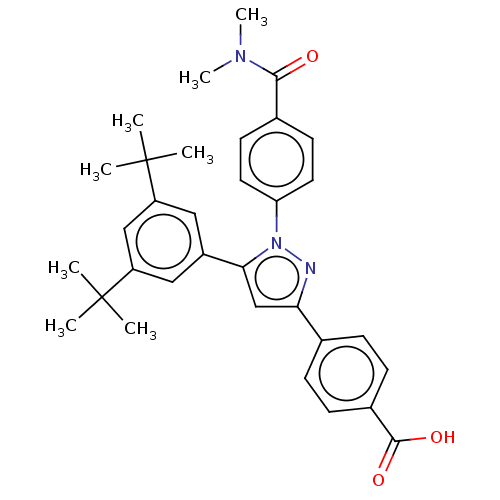
(CHEMBL3814385)Show SMILES CN(C)C(=O)c1ccc(cc1)-n1nc(cc1-c1cc(cc(c1)C(C)(C)C)C(C)(C)C)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C33H37N3O3/c1-32(2,3)25-17-24(18-26(19-25)33(4,5)6)29-20-28(21-9-11-23(12-10-21)31(38)39)34-36(29)27-15-13-22(14-16-27)30(37)35(7)8/h9-20H,1-8H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178960
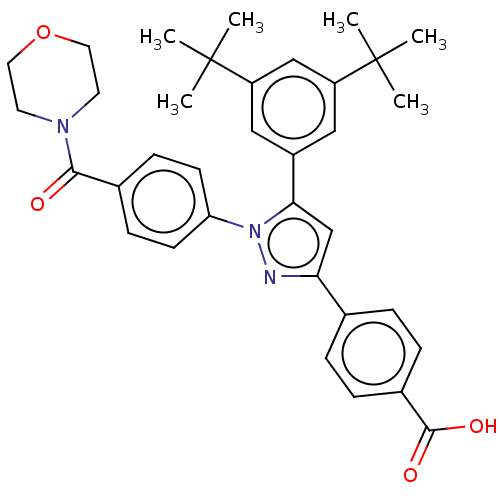
(CHEMBL3813702)Show SMILES CC(C)(C)c1cc(cc(c1)C(C)(C)C)-c1cc(nn1-c1ccc(cc1)C(=O)N1CCOCC1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C35H39N3O4/c1-34(2,3)27-19-26(20-28(21-27)35(4,5)6)31-22-30(23-7-9-25(10-8-23)33(40)41)36-38(31)29-13-11-24(12-14-29)32(39)37-15-17-42-18-16-37/h7-14,19-22H,15-18H2,1-6H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178958

(CHEMBL3813975)Show SMILES CC(C)(C)c1cc(cc(c1)C(C)(C)C)-c1cc(nn1-c1ccc(cc1)C(N)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C31H33N3O3/c1-30(2,3)23-15-22(16-24(17-23)31(4,5)6)27-18-26(19-7-9-21(10-8-19)29(36)37)33-34(27)25-13-11-20(12-14-25)28(32)35/h7-18H,1-6H3,(H2,32,35)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033806
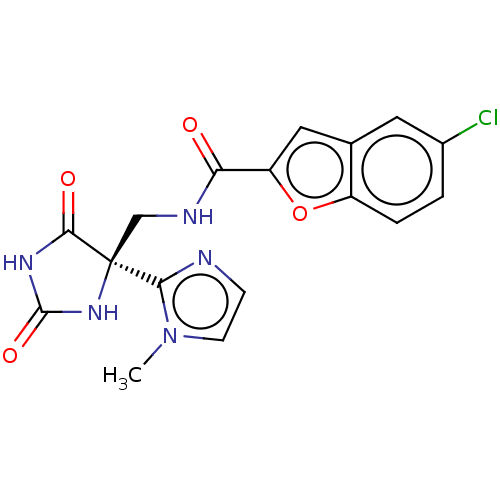
(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532313
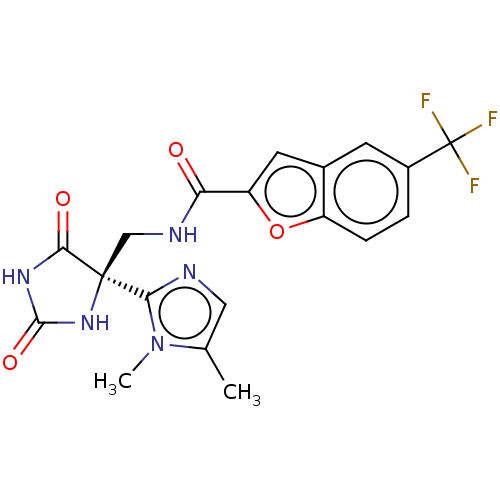
(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033806

(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033806

(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532313

(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033806

(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532312
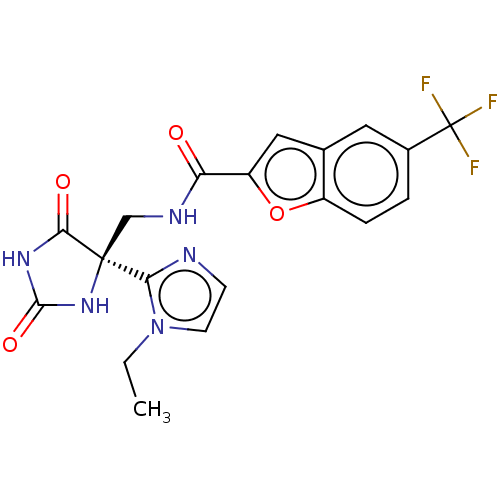
(CHEMBL4450729)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-2-27-6-5-23-15(27)18(16(29)25-17(30)26-18)9-24-14(28)13-8-10-7-11(19(20,21)22)3-4-12(10)31-13/h3-8H,2,9H2,1H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033808
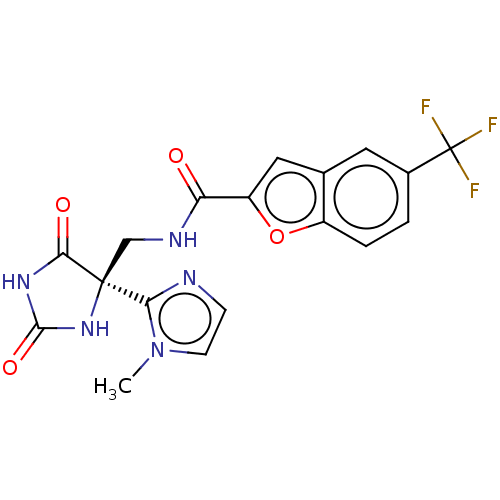
(CHEMBL3358158)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H14F3N5O4/c1-26-5-4-22-14(26)17(15(28)24-16(29)25-17)8-23-13(27)12-7-9-6-10(18(19,20)21)2-3-11(9)30-12/h2-7H,8H2,1H3,(H,23,27)(H2,24,25,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532312

(CHEMBL4450729)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-2-27-6-5-23-15(27)18(16(29)25-17(30)26-18)9-24-14(28)13-8-10-7-11(19(20,21)22)3-4-12(10)31-13/h3-8H,2,9H2,1H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033808

(CHEMBL3358158)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H14F3N5O4/c1-26-5-4-22-14(26)17(15(28)24-16(29)25-17)8-23-13(27)12-7-9-6-10(18(19,20)21)2-3-11(9)30-12/h2-7H,8H2,1H3,(H,23,27)(H2,24,25,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033808

(CHEMBL3358158)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H14F3N5O4/c1-26-5-4-22-14(26)17(15(28)24-16(29)25-17)8-23-13(27)12-7-9-6-10(18(19,20)21)2-3-11(9)30-12/h2-7H,8H2,1H3,(H,23,27)(H2,24,25,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532312

(CHEMBL4450729)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-2-27-6-5-23-15(27)18(16(29)25-17(30)26-18)9-24-14(28)13-8-10-7-11(19(20,21)22)3-4-12(10)31-13/h3-8H,2,9H2,1H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033808

(CHEMBL3358158)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H14F3N5O4/c1-26-5-4-22-14(26)17(15(28)24-16(29)25-17)8-23-13(27)12-7-9-6-10(18(19,20)21)2-3-11(9)30-12/h2-7H,8H2,1H3,(H,23,27)(H2,24,25,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532312

(CHEMBL4450729)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-2-27-6-5-23-15(27)18(16(29)25-17(30)26-18)9-24-14(28)13-8-10-7-11(19(20,21)22)3-4-12(10)31-13/h3-8H,2,9H2,1H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532313

(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532313

(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50238243
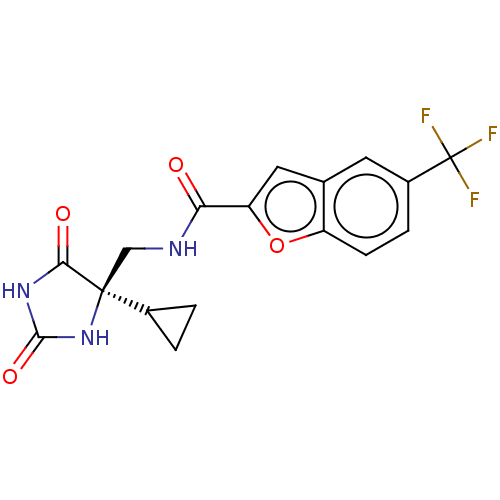
(CHEMBL4097165)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C17H14F3N3O4/c18-17(19,20)10-3-4-11-8(5-10)6-12(27-11)13(24)21-7-16(9-1-2-9)14(25)22-15(26)23-16/h3-6,9H,1-2,7H2,(H,21,24)(H2,22,23,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50238243

(CHEMBL4097165)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C17H14F3N3O4/c18-17(19,20)10-3-4-11-8(5-10)6-12(27-11)13(24)21-7-16(9-1-2-9)14(25)22-15(26)23-16/h3-6,9H,1-2,7H2,(H,21,24)(H2,22,23,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 1
(Homo sapiens (Human)) | BDBM50535848
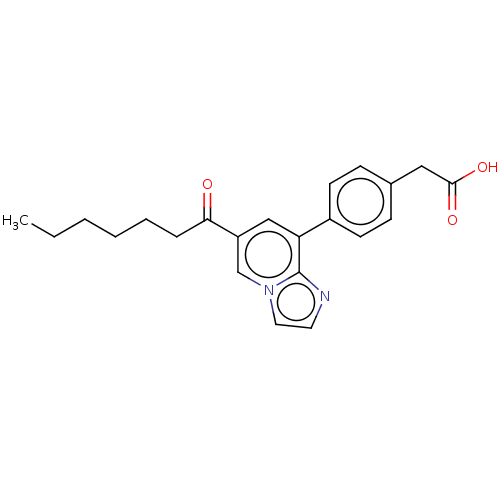
(CHEMBL4584997)Show SMILES CCCCCCC(=O)c1cc(-c2ccc(CC(O)=O)cc2)c2nccn2c1 Show InChI InChI=1S/C22H24N2O3/c1-2-3-4-5-6-20(25)18-14-19(22-23-11-12-24(22)15-18)17-9-7-16(8-10-17)13-21(26)27/h7-12,14-15H,2-6,13H2,1H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human SPT1 expressed in microsomes of HEK293 cells incubated for 1hr by LC/MS analysis |
J Med Chem 59: 5904-10 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01851
BindingDB Entry DOI: 10.7270/Q29K4FR1 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532311
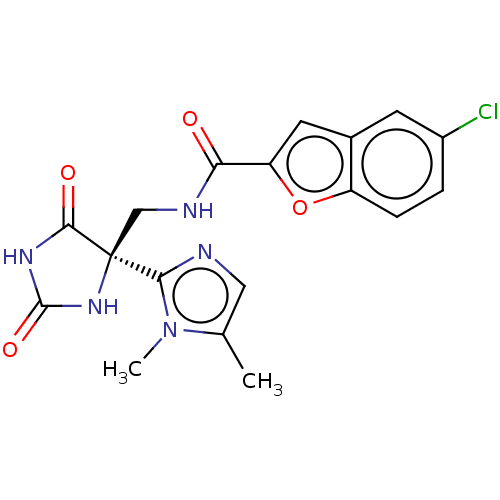
(CHEMBL4587825)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-9-7-20-15(24(9)2)18(16(26)22-17(27)23-18)8-21-14(25)13-6-10-5-11(19)3-4-12(10)28-13/h3-7H,8H2,1-2H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532311

(CHEMBL4587825)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-9-7-20-15(24(9)2)18(16(26)22-17(27)23-18)8-21-14(25)13-6-10-5-11(19)3-4-12(10)28-13/h3-7H,8H2,1-2H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50238243

(CHEMBL4097165)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C17H14F3N3O4/c18-17(19,20)10-3-4-11-8(5-10)6-12(27-11)13(24)21-7-16(9-1-2-9)14(25)22-15(26)23-16/h3-6,9H,1-2,7H2,(H,21,24)(H2,22,23,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50238243

(CHEMBL4097165)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C17H14F3N3O4/c18-17(19,20)10-3-4-11-8(5-10)6-12(27-11)13(24)21-7-16(9-1-2-9)14(25)22-15(26)23-16/h3-6,9H,1-2,7H2,(H,21,24)(H2,22,23,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532311

(CHEMBL4587825)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-9-7-20-15(24(9)2)18(16(26)22-17(27)23-18)8-21-14(25)13-6-10-5-11(19)3-4-12(10)28-13/h3-7H,8H2,1-2H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532311

(CHEMBL4587825)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-9-7-20-15(24(9)2)18(16(26)22-17(27)23-18)8-21-14(25)13-6-10-5-11(19)3-4-12(10)28-13/h3-7H,8H2,1-2H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532310
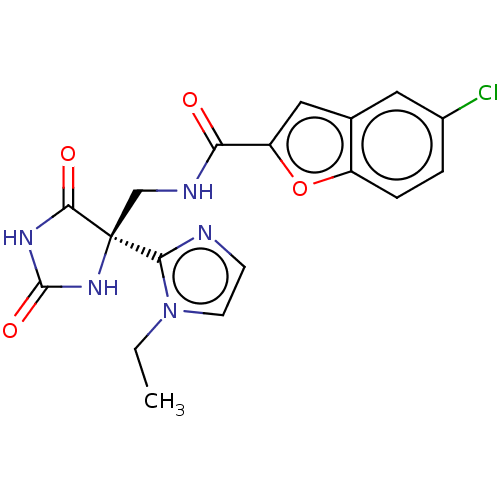
(CHEMBL4589438)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-2-24-6-5-20-15(24)18(16(26)22-17(27)23-18)9-21-14(25)13-8-10-7-11(19)3-4-12(10)28-13/h3-8H,2,9H2,1H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033805
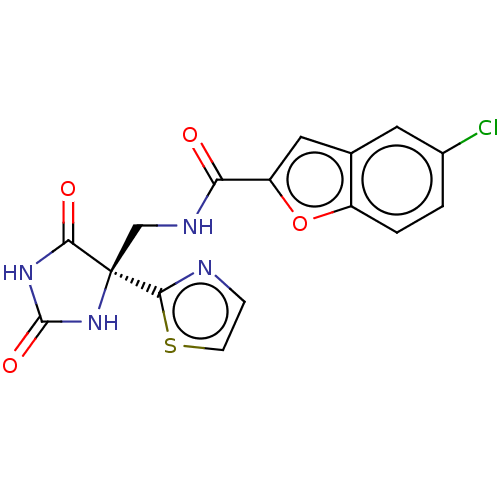
(CHEMBL3358155)Show SMILES Clc1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)c1nccs1 |r| Show InChI InChI=1S/C16H11ClN4O4S/c17-9-1-2-10-8(5-9)6-11(25-10)12(22)19-7-16(14-18-3-4-26-14)13(23)20-15(24)21-16/h1-6H,7H2,(H,19,22)(H2,20,21,23,24)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033805

(CHEMBL3358155)Show SMILES Clc1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)c1nccs1 |r| Show InChI InChI=1S/C16H11ClN4O4S/c17-9-1-2-10-8(5-9)6-11(25-10)12(22)19-7-16(14-18-3-4-26-14)13(23)20-15(24)21-16/h1-6H,7H2,(H,19,22)(H2,20,21,23,24)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532310

(CHEMBL4589438)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-2-24-6-5-20-15(24)18(16(26)22-17(27)23-18)9-21-14(25)13-8-10-7-11(19)3-4-12(10)28-13/h3-8H,2,9H2,1H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data