 Found 76 hits with Last Name = 'schutt' and Initial = 'lk'
Found 76 hits with Last Name = 'schutt' and Initial = 'lk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572808
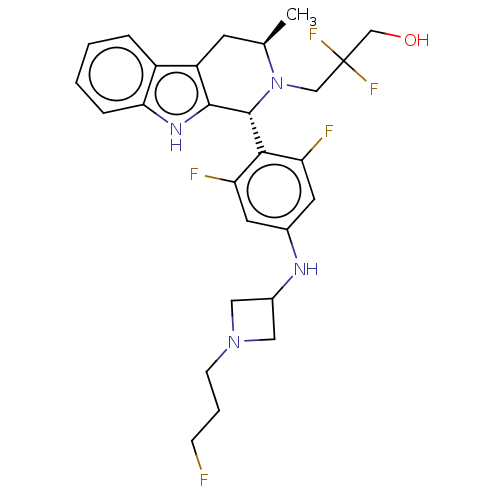
(Gdc-9545 | Giredestrant | RO-7197597 | RO7197597)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NC2CN(CCCF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572809
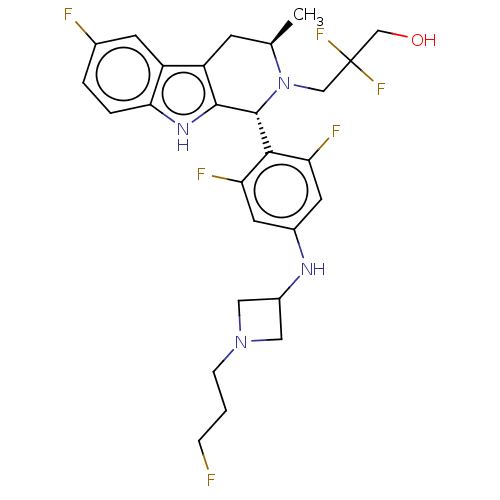
(CHEMBL4866043)Show SMILES C[C@@H]1Cc2c([nH]c3ccc(F)cc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NC2CN(CCCF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572829
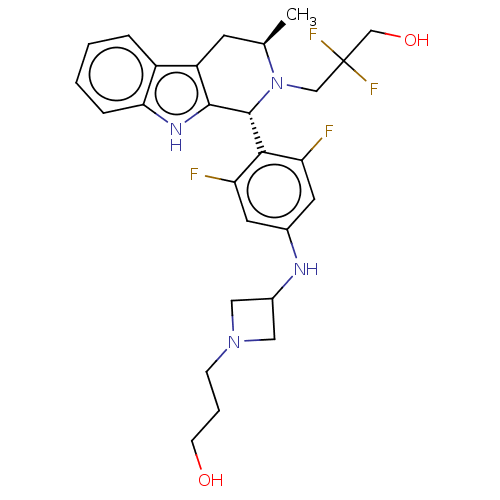
(CHEMBL4856969)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NC2CN(CCCO)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572825
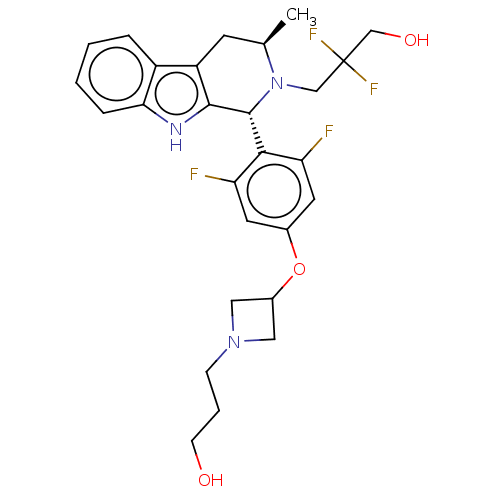
(CHEMBL4856892)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(OC2CN(CCCO)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572826
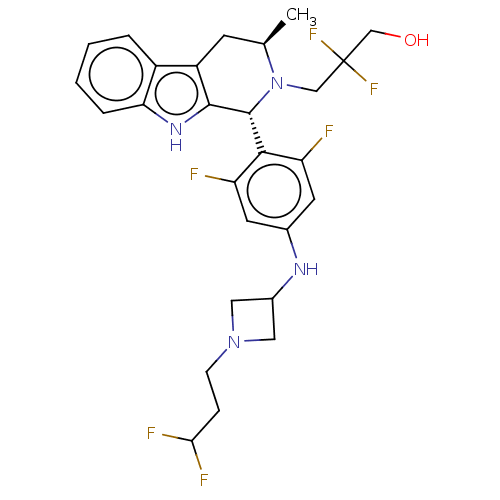
(CHEMBL4869698)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NC2CN(CCC(F)F)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572832
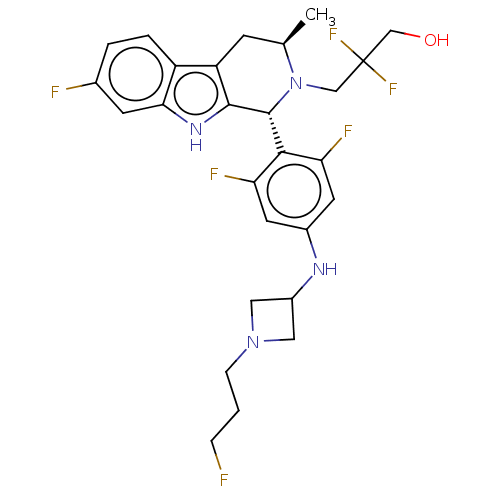
(CHEMBL4845726)Show SMILES C[C@@H]1Cc2c([nH]c3cc(F)ccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NC2CN(CCCF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572828
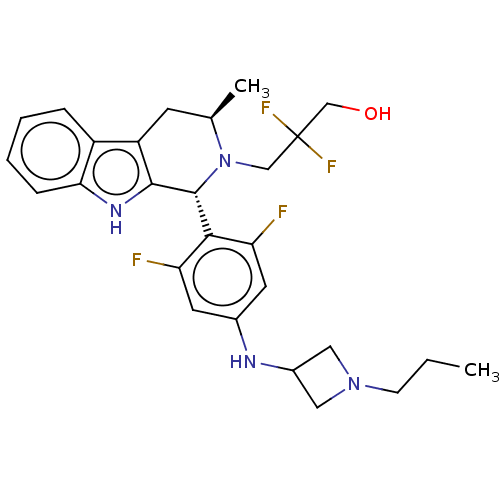
(CHEMBL4864829)Show SMILES CCCN1CC(C1)Nc1cc(F)c([C@H]2N(CC(F)(F)CO)[C@H](C)Cc3c2[nH]c2ccccc32)c(F)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM368199
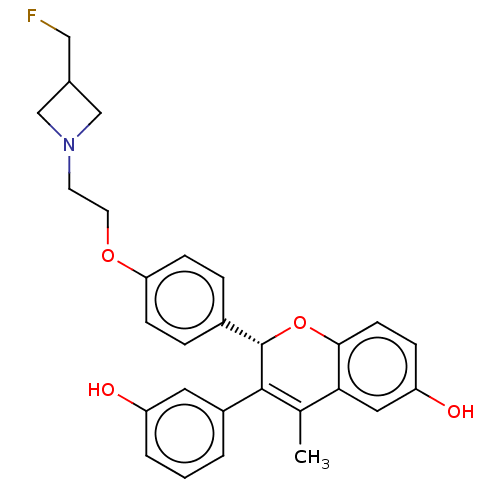
((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572821
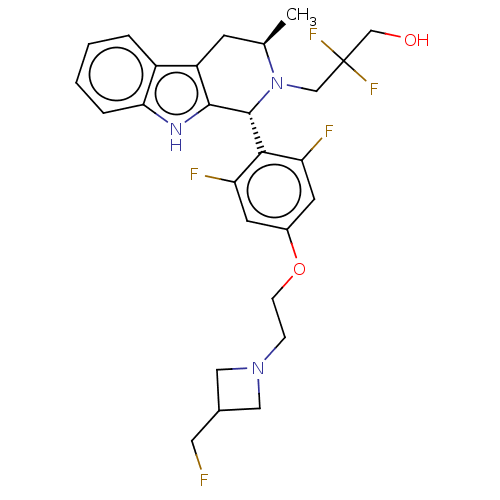
(CHEMBL4871161)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572824
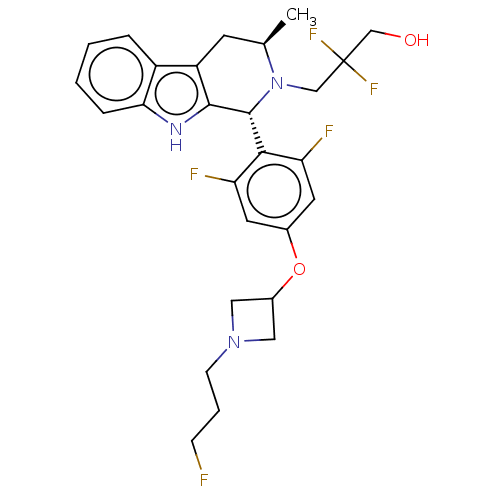
(CHEMBL4857736)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(OC2CN(CCCF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50542086
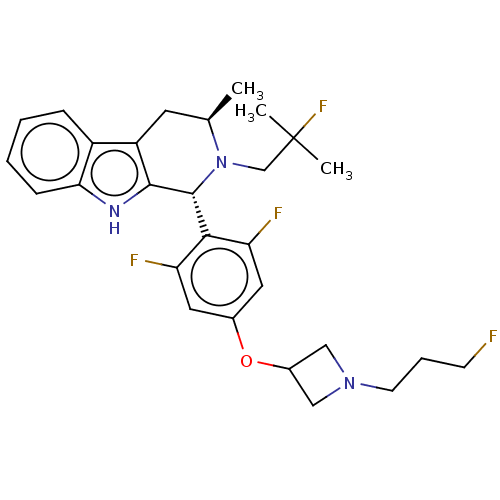
(CHEMBL4649161)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(OC2CN(CCCF)C2)cc1F |r| Show InChI InChI=1S/C28H33F4N3O/c1-17-11-21-20-7-4-5-8-24(20)33-26(21)27(35(17)16-28(2,3)32)25-22(30)12-18(13-23(25)31)36-19-14-34(15-19)10-6-9-29/h4-5,7-8,12-13,17,19,27,33H,6,9-11,14-16H2,1-3H3/t17-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572831
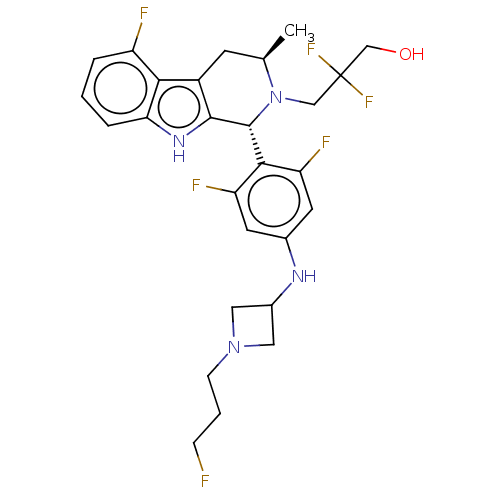
(CHEMBL4877338)Show SMILES C[C@@H]1Cc2c([nH]c3cccc(F)c23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NC2CN(CCCF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572834
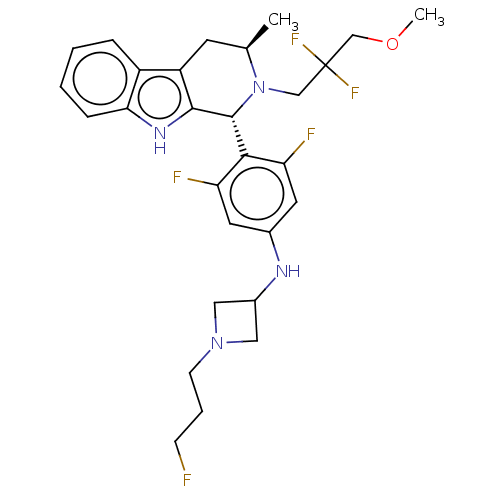
(CHEMBL4853118)Show SMILES COCC(F)(F)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(NC2CN(CCCF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572823
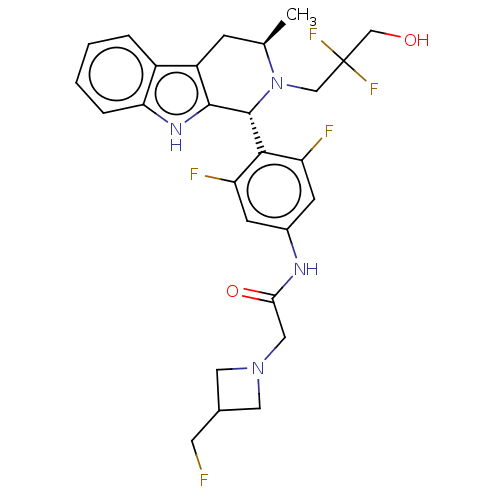
(CHEMBL4860671)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NC(=O)CN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM299111

(US10125135, Example 1)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(C)(C)F)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572822
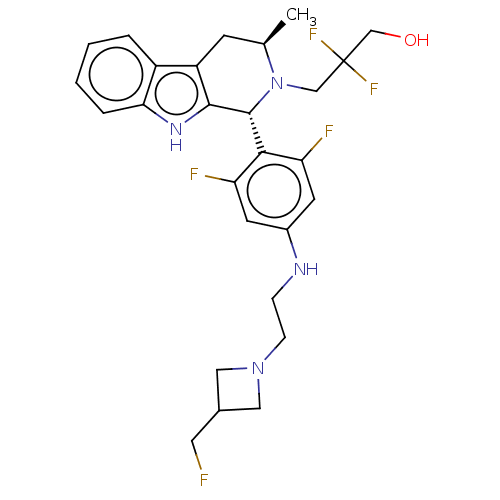
(CHEMBL4866232)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572833
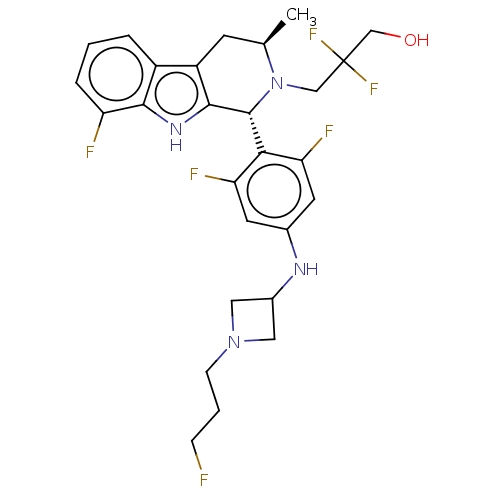
(CHEMBL4873860)Show SMILES C[C@@H]1Cc2c([nH]c3c(F)cccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NC2CN(CCCF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50238741
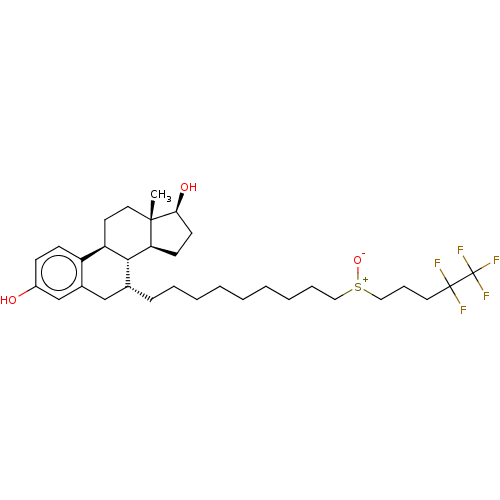
(CHEBI:31638 | Faslodex | Fulvestrant | ICI-182780 ...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3C[C@@H](CCCCCCCCC[S+]([O-])CCCC(F)(F)C(F)(F)F)[C@@]21[H] Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572827
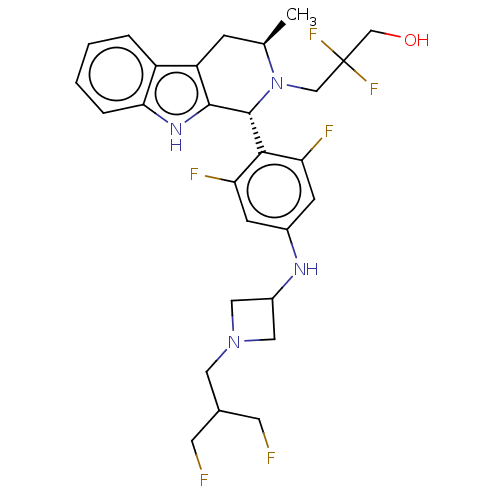
(CHEMBL4871601)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(NC2CN(CC(CF)CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572815
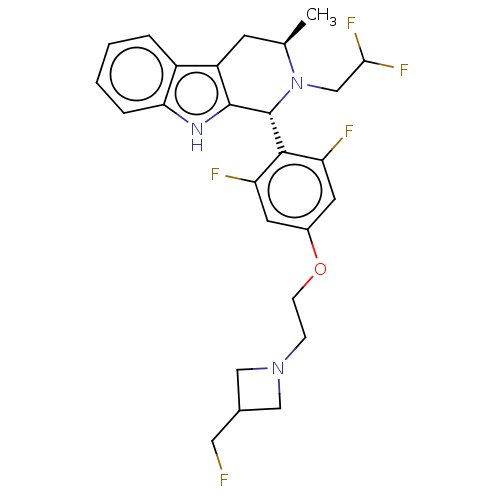
(CHEMBL4847707)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)F)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572820
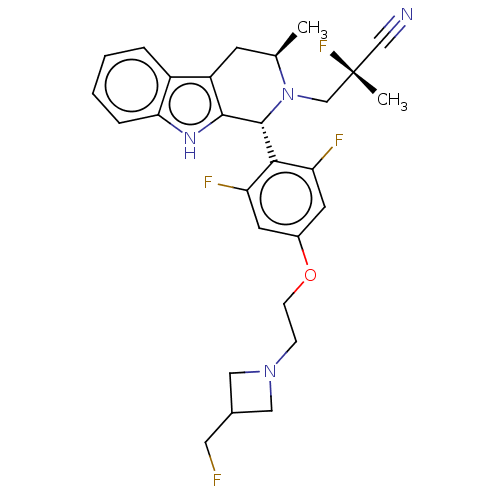
(CHEMBL4850919)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1C[C@](C)(F)C#N)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572817
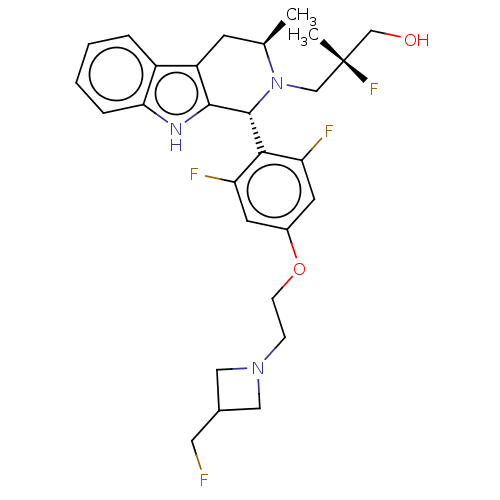
(CHEMBL4854930)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1C[C@@](C)(F)CO)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572814

(CHEMBL4867106)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC1(C)COC1)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50503111
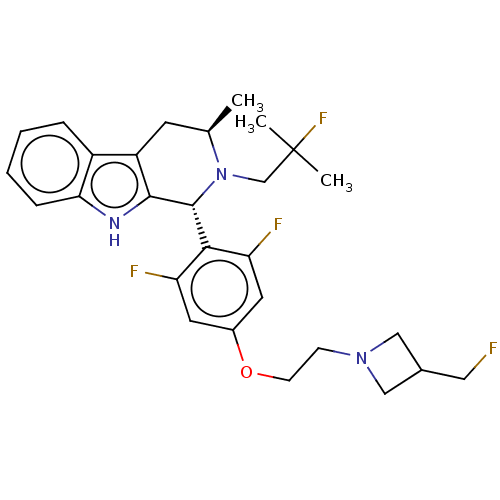
(CHEMBL4528514 | US11672785, Goodacre Compound 102 ...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| Show InChI InChI=1S/C28H33F4N3O/c1-17-10-21-20-6-4-5-7-24(20)33-26(21)27(35(17)16-28(2,3)32)25-22(30)11-19(12-23(25)31)36-9-8-34-14-18(13-29)15-34/h4-7,11-12,17-18,27,33H,8-10,13-16H2,1-3H3/t17-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572836
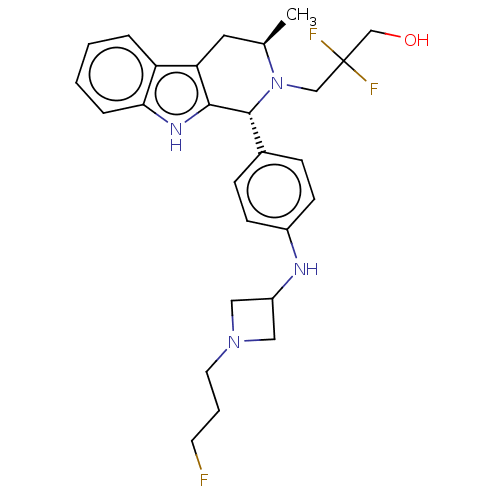
(CHEMBL4877406)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1ccc(NC2CN(CCCF)C2)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572812
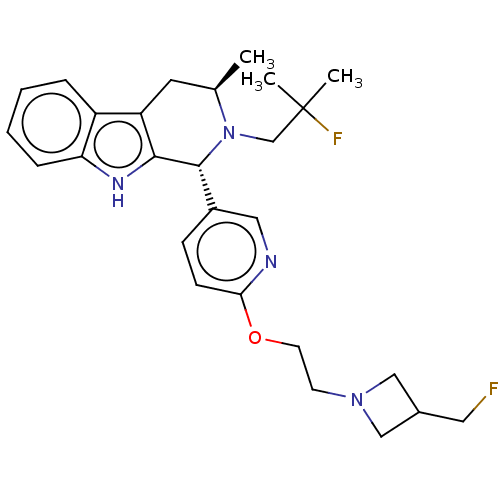
(CHEMBL4852984)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(OCCN2CC(CF)C2)nc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572816
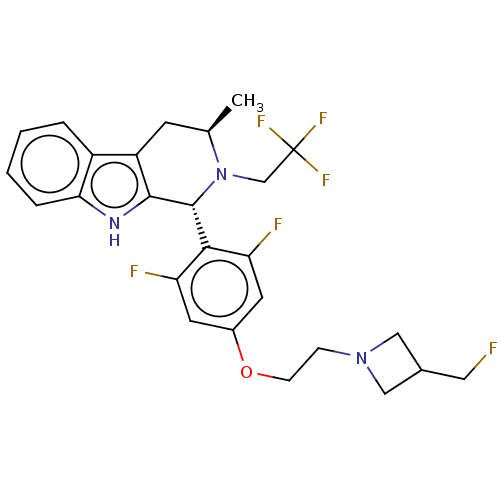
(CHEMBL4858543)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)F)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572818
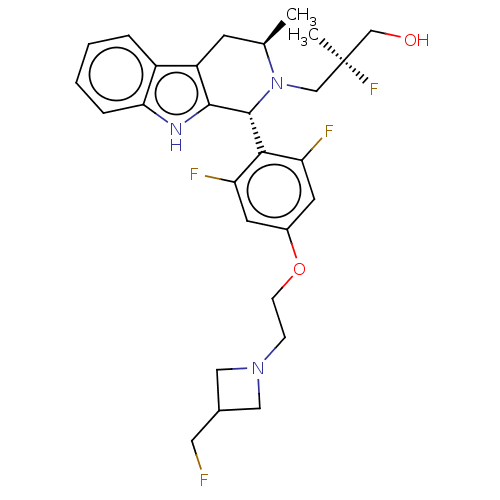
(CHEMBL4847664)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1C[C@](C)(F)CO)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572830
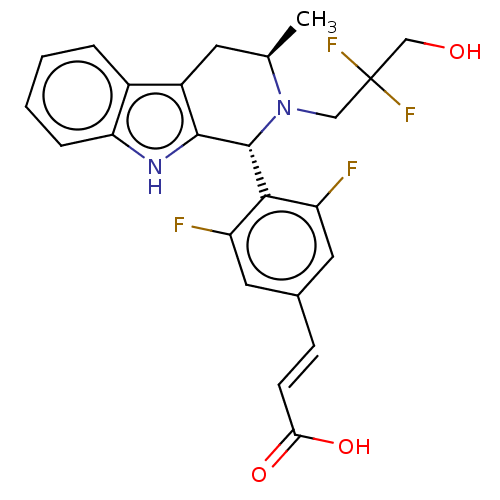
(CHEMBL4864337)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1c(F)cc(\C=C\C(O)=O)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572835
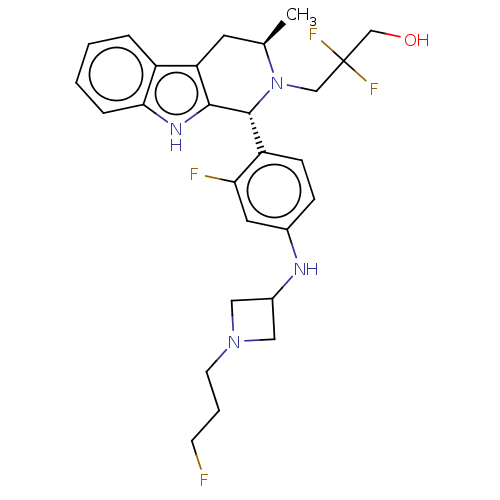
(CHEMBL4865515)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(F)(F)CO)c1ccc(NC2CN(CCCF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
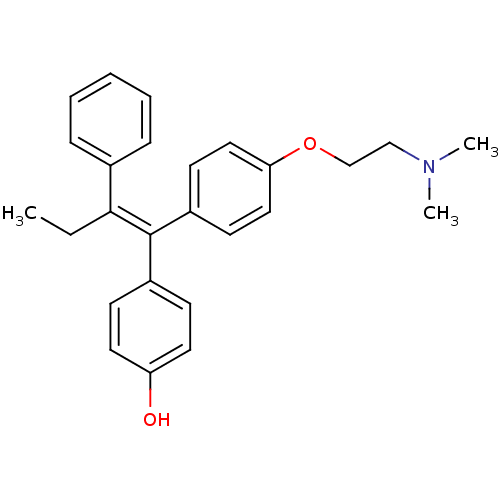
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597817
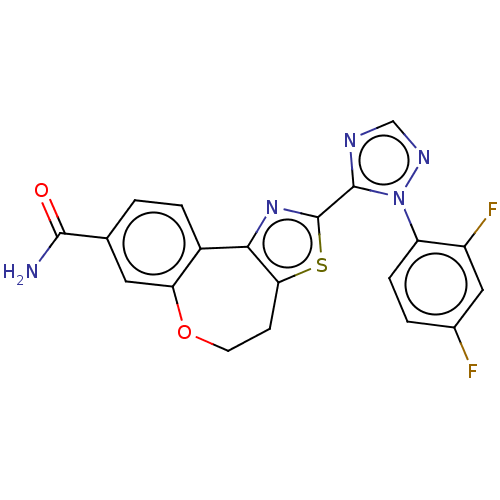
(CHEMBL5192406)Show SMILES NC(=O)c1ccc2-c3nc(sc3CCOc2c1)-c1ncnn1-c1ccc(F)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572819
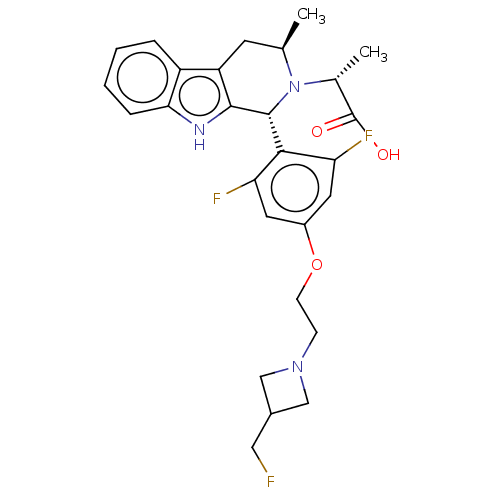
(CHEMBL4864628)Show SMILES C[C@@H](N1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(OCCN2CC(CF)C2)cc1F)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597812
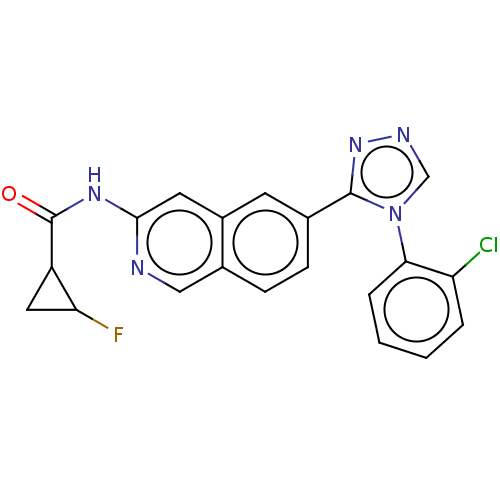
(CHEMBL5192215)Show SMILES FC1CC1C(=O)Nc1cc2cc(ccc2cn1)-c1nncn1-c1ccccc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50597817

(CHEMBL5192406)Show SMILES NC(=O)c1ccc2-c3nc(sc3CCOc2c1)-c1ncnn1-c1ccc(F)cc1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572810
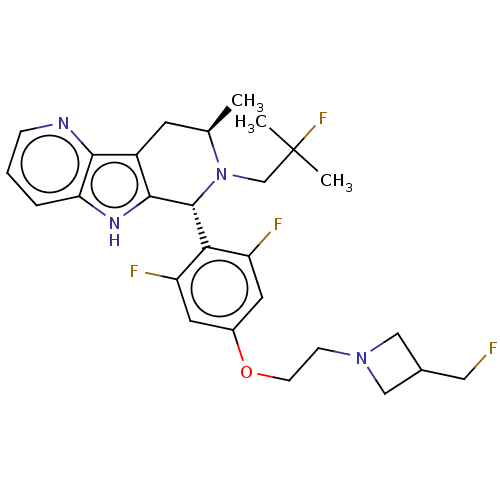
(CHEMBL4854999)Show SMILES C[C@@H]1Cc2c([nH]c3cccnc23)[C@H](N1CC(C)(C)F)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50090462
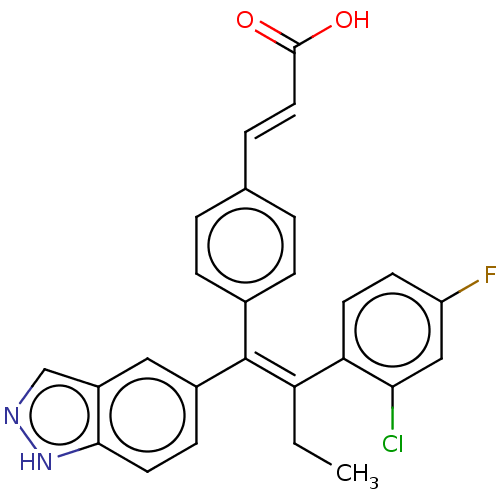
(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572813
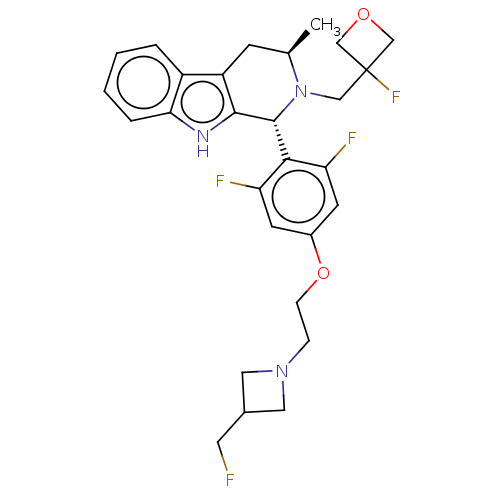
(CHEMBL4862431)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC1(F)COC1)c1c(F)cc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50572811

(CHEMBL4869612)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ncc(OCCN2CC(CF)C2)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597813

(CHEMBL5183804)Show SMILES CC(C)[C@@H]1CNC(=O)c2cc(nn12)-c1ccnc(NC(=O)C2CC2)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597807
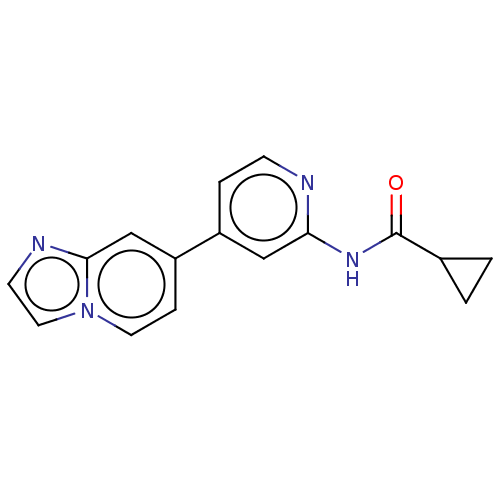
(CHEMBL5190688) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548269
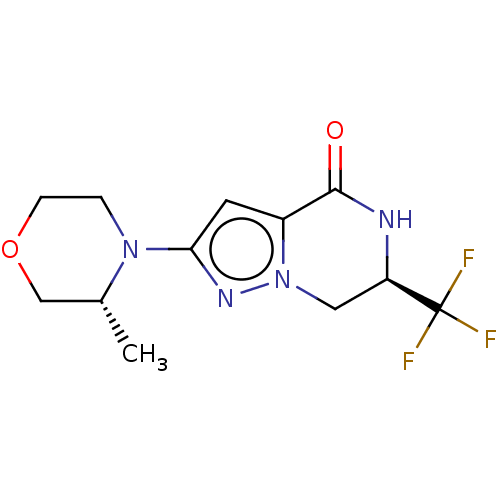
((R)-2-((R)-3-methylmorpholino)-6-(trifluoromethyl)...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)N[C@H](Cn2n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548239
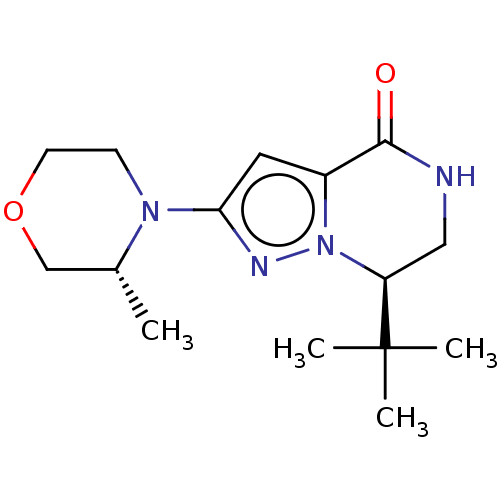
((R)-7-(tert-butyl)-2-((R)-3-methylmorpholino)-6,7-...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC[C@H](n2n1)C(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597814
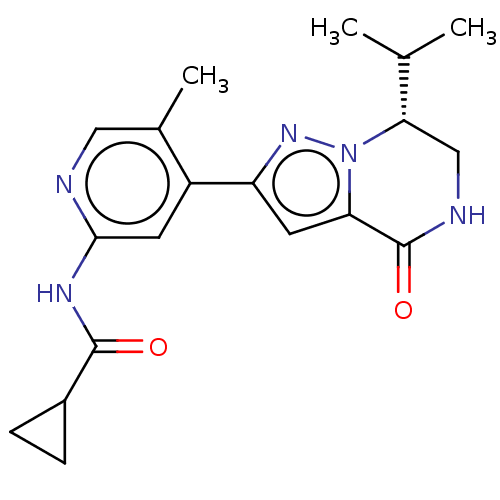
(CHEMBL5200373)Show SMILES CC(C)[C@@H]1CNC(=O)c2cc(nn12)-c1cc(NC(=O)C2CC2)ncc1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597809
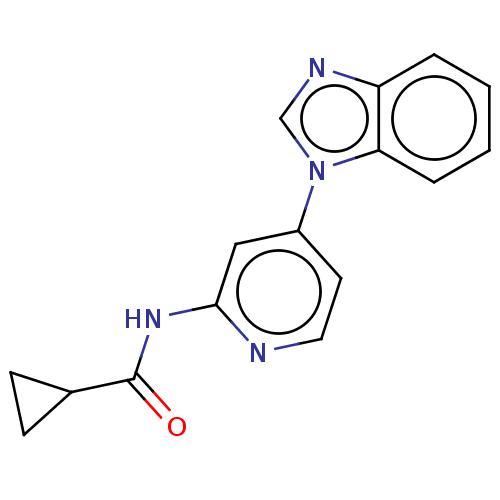
(CHEMBL5181274) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20607
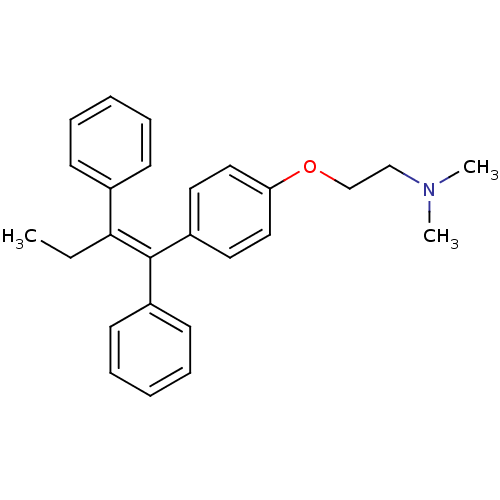
((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00847
BindingDB Entry DOI: 10.7270/Q20R9T67 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548235
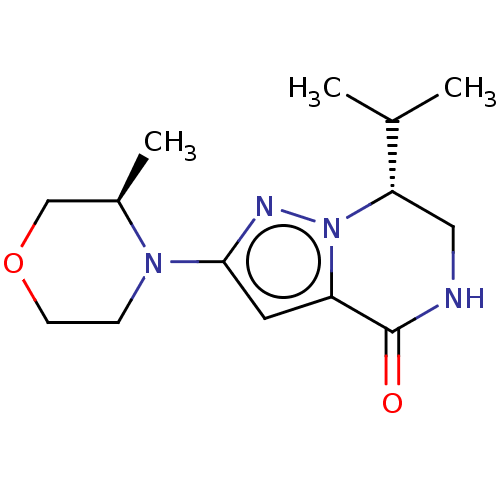
((R)-7-isopropyl-2-((R)-3-methylmorpholino)-6,7-dih...)Show SMILES CC(C)[C@@H]1CNC(=O)c2cc(nn12)N1CCOC[C@H]1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548243
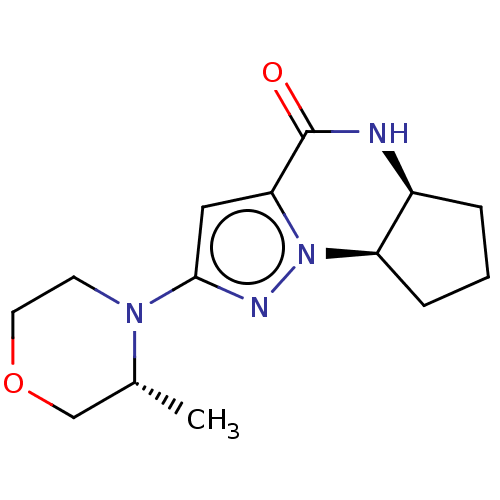
((5aS,8aR)-2-((R)-3-methylmorpholino)-5,5a,6,7,8,8a...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)N[C@H]3CCC[C@H]3n2n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548265
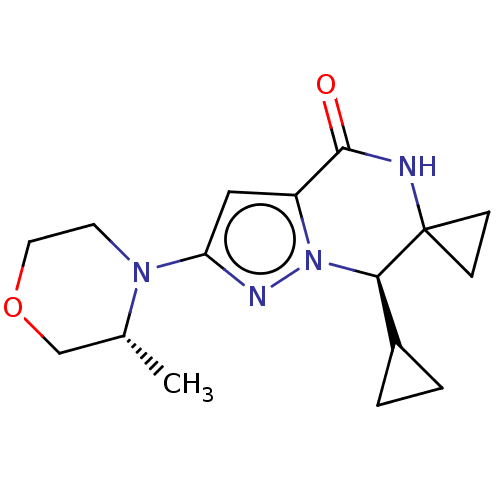
((R)-7'-cyclopropyl-2'-((R)-3-methylmorpholino)-7'H...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC3(CC3)[C@@H](C3CC3)n2n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548251
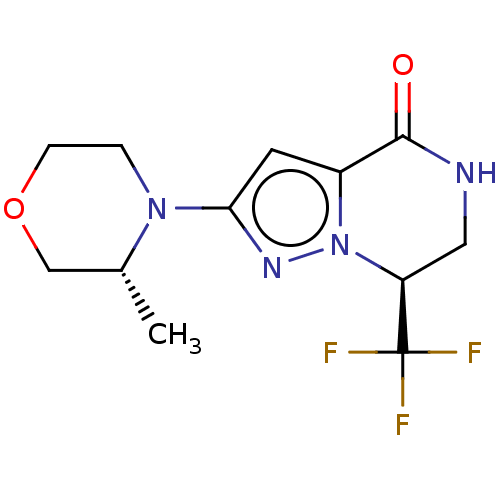
((S)-2-((R)-3-methylmorpholino)-7-(trifluoromethyl)...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC[C@H](n2n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data