 Found 28 hits with Last Name = 'sandjo' and Initial = 'lp'
Found 28 hits with Last Name = 'sandjo' and Initial = 'lp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590189
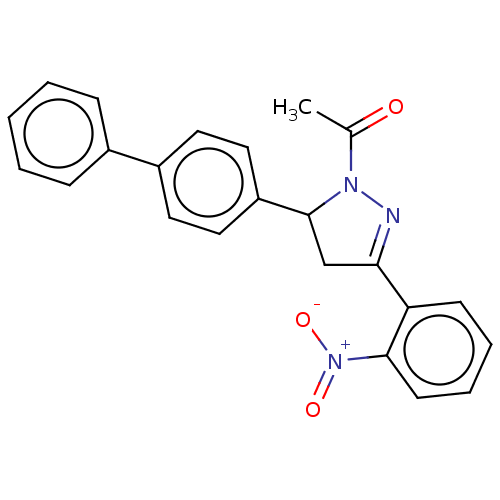
(CHEMBL5191187)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)-c1ccccc1)c1ccccc1[N+]([O-])=O |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590196
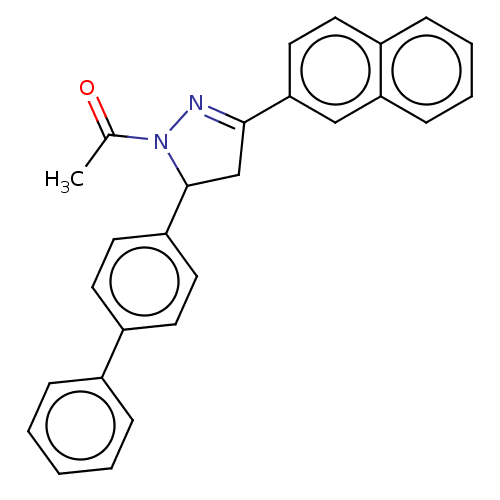
(CHEMBL5187436)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)-c1ccccc1)c1ccc2ccccc2c1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590180
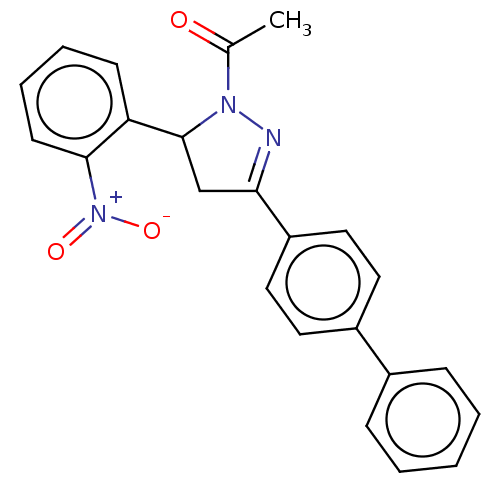
(CHEMBL5179832)Show SMILES CC(=O)N1N=C(CC1c1ccccc1[N+]([O-])=O)c1ccc(cc1)-c1ccccc1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590187
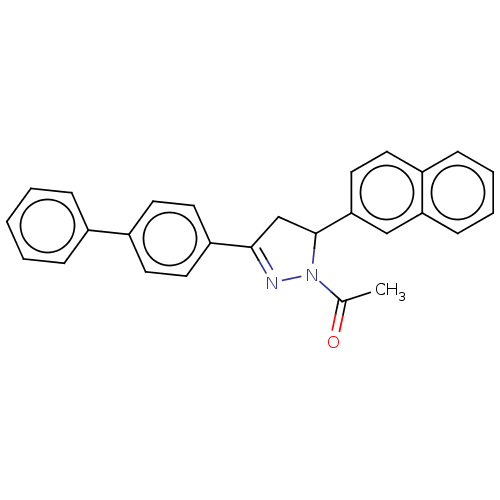
(CHEMBL5197832)Show SMILES CC(=O)N1N=C(CC1c1ccc2ccccc2c1)c1ccc(cc1)-c1ccccc1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 799 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590188
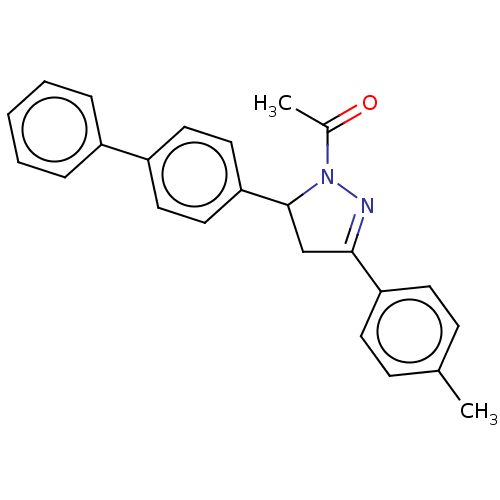
(CHEMBL5189505)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)-c1ccccc1)c1ccc(C)cc1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 845 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590190

(CHEMBL5177419)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)-c1ccccc1)c1ccc(Br)cc1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 903 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590194
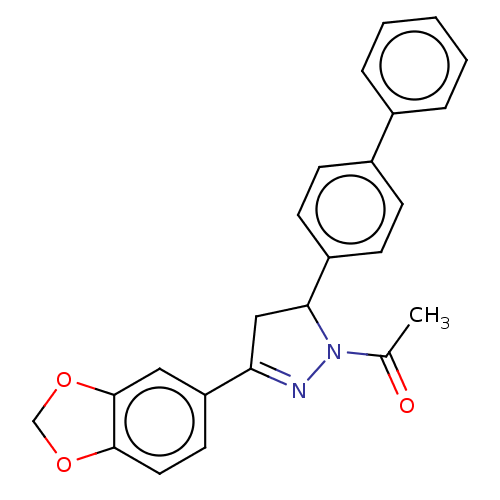
(CHEMBL5184984)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)-c1ccccc1)c1ccc2OCOc2c1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 912 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590186

(CHEMBL5188500)Show SMILES CC(=O)N1N=C(CC1c1ccc2OCOc2c1)c1ccc(cc1)-c1ccccc1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 988 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 1-alpha/beta
(Homo sapiens (Human)) | BDBM50207369

(CHEMBL3968323)Show SMILES [H][C@]12O[C@@]11[C@]([H])(C[C@]34CC[C@H](C=C)[C@@]3(C)[C@@H](O)C[C@]14C)[C@H](C)C2=O |r| Show InChI InChI=1S/C19H26O3/c1-5-11-6-7-18-8-12-10(2)14(21)15-19(12,22-15)16(18,3)9-13(20)17(11,18)4/h5,10-13,15,20H,1,6-9H2,2-4H3/t10-,11-,12+,13-,15+,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern
Curated by ChEMBL
| Assay Description
Inhibition of Stat1 (unknown origin) expressed in LPS/INF-gamma-stimulated human MONO-MAC-6 cells assessed as reduction in GAS dependent transcriptio... |
Bioorg Med Chem 25: 514-522 (2017)
Article DOI: 10.1016/j.bmc.2016.11.016
BindingDB Entry DOI: 10.7270/Q2P2713X |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 1-alpha/beta
(Homo sapiens (Human)) | BDBM50207367
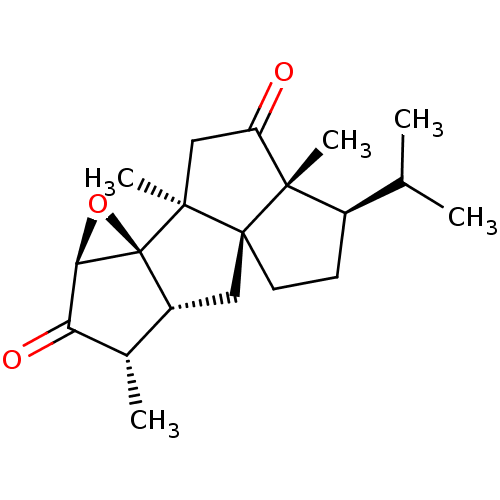
(CHEMBL3952820)Show SMILES [H][C@]12O[C@@]11[C@]([H])(C[C@]34CC[C@H](C(C)C)[C@@]3(C)C(=O)C[C@]14C)[C@H](C)C2=O |r| Show InChI InChI=1S/C20H28O3/c1-10(2)12-6-7-19-8-13-11(3)15(22)16-20(13,23-16)17(19,4)9-14(21)18(12,19)5/h10-13,16H,6-9H2,1-5H3/t11-,12+,13+,16+,17-,18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern
Curated by ChEMBL
| Assay Description
Inhibition of Stat1 (unknown origin) expressed in LPS/INF-gamma-stimulated human MONO-MAC-6 cells assessed as reduction in GAS dependent transcriptio... |
Bioorg Med Chem 25: 514-522 (2017)
Article DOI: 10.1016/j.bmc.2016.11.016
BindingDB Entry DOI: 10.7270/Q2P2713X |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 1-alpha/beta
(Homo sapiens (Human)) | BDBM50207368

(CHEMBL3904582)Show SMILES [H][C@]12O[C@@]11[C@]([H])(C[C@]34CC[C@H](C(C)C)[C@@]3(C)C(=O)[C@H](O)[C@]14C)[C@H](C)C2=O |r| Show InChI InChI=1S/C20H28O4/c1-9(2)11-6-7-19-8-12-10(3)13(21)16-20(12,24-16)18(19,5)15(23)14(22)17(11,19)4/h9-12,15-16,23H,6-8H2,1-5H3/t10-,11+,12+,15-,16+,17-,18-,19+,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern
Curated by ChEMBL
| Assay Description
Inhibition of Stat1 (unknown origin) expressed in LPS/INF-gamma-stimulated human MONO-MAC-6 cells assessed as reduction in GAS dependent transcriptio... |
Bioorg Med Chem 25: 514-522 (2017)
Article DOI: 10.1016/j.bmc.2016.11.016
BindingDB Entry DOI: 10.7270/Q2P2713X |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 1-alpha/beta
(Homo sapiens (Human)) | BDBM50016098
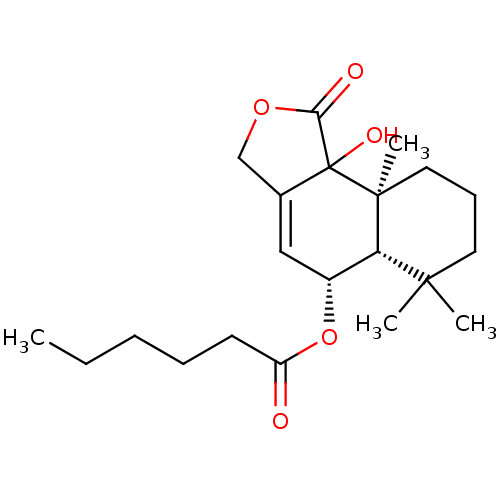
(CHEMBL3261195)Show SMILES [H][C@@]12[C@H](OC(=O)CCCCC)C=C3COC(=O)C3(O)[C@@]1(C)CCCC2(C)C |r,t:11| Show InChI InChI=1S/C21H32O5/c1-5-6-7-9-16(22)26-15-12-14-13-25-18(23)21(14,24)20(4)11-8-10-19(2,3)17(15)20/h12,15,17,24H,5-11,13H2,1-4H3/t15-,17+,20+,21?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology and Drug Research (IBWF)
Curated by ChEMBL
| Assay Description
Inhibition of Stat1 (unknown origin)-induced human CXCL10 (972 bp) promoter activity expressed in TNF-alpha/IFN-gamma/IL-1beta-stimulated human DLD1 ... |
Bioorg Med Chem 22: 2912-8 (2014)
Article DOI: 10.1016/j.bmc.2014.04.015
BindingDB Entry DOI: 10.7270/Q21J9CBW |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 1-alpha/beta
(Homo sapiens (Human)) | BDBM50207366
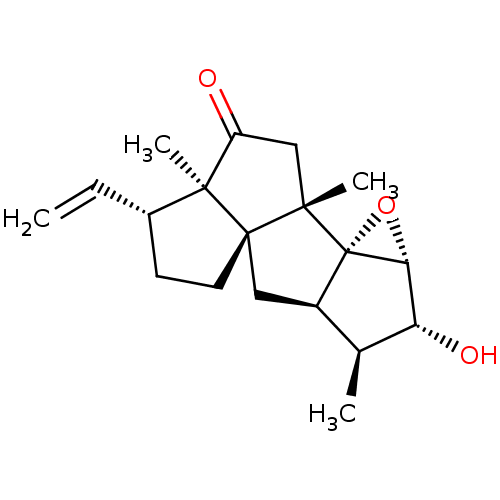
(CHEMBL3938768)Show SMILES [H][C@]12O[C@@]11[C@]([H])(C[C@]34CC[C@H](C=C)[C@@]3(C)C(=O)C[C@]14C)[C@H](C)[C@H]2O |r| Show InChI InChI=1S/C19H26O3/c1-5-11-6-7-18-8-12-10(2)14(21)15-19(12,22-15)16(18,3)9-13(20)17(11,18)4/h5,10-12,14-15,21H,1,6-9H2,2-4H3/t10-,11-,12+,14+,15+,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern
Curated by ChEMBL
| Assay Description
Inhibition of Stat1 (unknown origin) expressed in LPS/INF-gamma-stimulated human MONO-MAC-6 cells assessed as reduction in GAS dependent transcriptio... |
Bioorg Med Chem 25: 514-522 (2017)
Article DOI: 10.1016/j.bmc.2016.11.016
BindingDB Entry DOI: 10.7270/Q2P2713X |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 1-alpha/beta
(Homo sapiens (Human)) | BDBM50016099
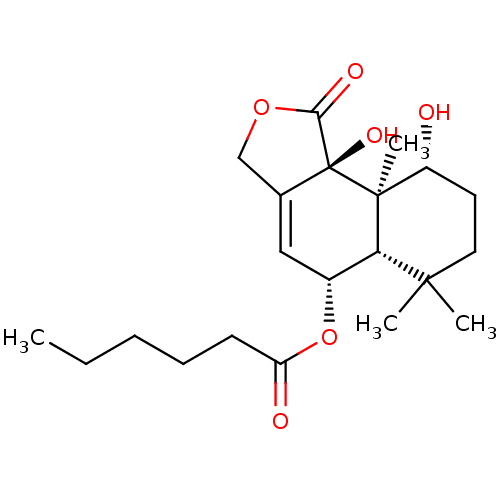
(CHEMBL3261196)Show SMILES [H][C@@]12[C@H](OC(=O)CCCCC)C=C3COC(=O)[C@]3(O)[C@@]1(C)[C@H](O)CCC2(C)C |r,t:11| Show InChI InChI=1S/C21H32O6/c1-5-6-7-8-16(23)27-14-11-13-12-26-18(24)21(13,25)20(4)15(22)9-10-19(2,3)17(14)20/h11,14-15,17,22,25H,5-10,12H2,1-4H3/t14-,15-,17+,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology and Drug Research (IBWF)
Curated by ChEMBL
| Assay Description
Inhibition of Stat1 (unknown origin)-induced human CXCL10 (972 bp) promoter activity expressed in TNF-alpha/IFN-gamma/IL-1beta-stimulated human DLD1 ... |
Bioorg Med Chem 22: 2912-8 (2014)
Article DOI: 10.1016/j.bmc.2014.04.015
BindingDB Entry DOI: 10.7270/Q21J9CBW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590195
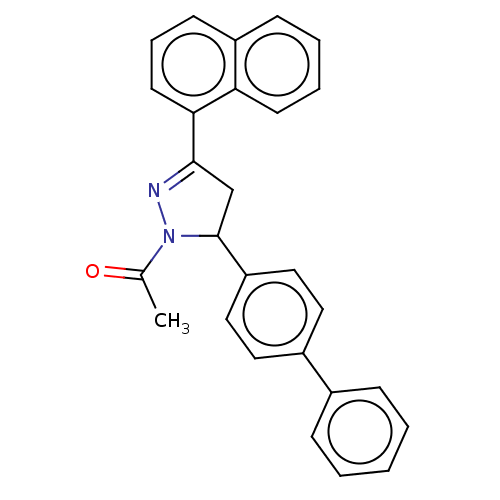
(CHEMBL5185580)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)-c1ccccc1)c1cccc2ccccc12 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590181
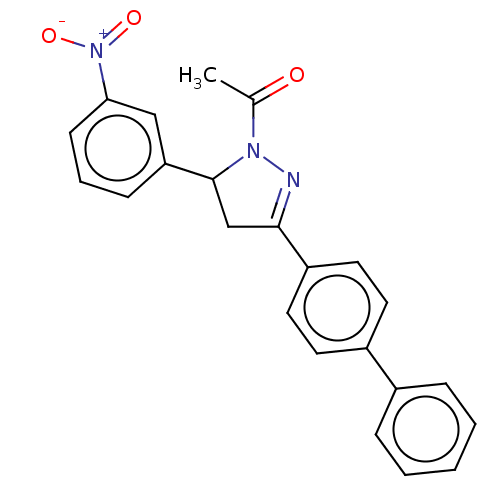
(CHEMBL5175686)Show SMILES CC(=O)N1N=C(CC1c1cccc(c1)[N+]([O-])=O)c1ccc(cc1)-c1ccccc1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590192
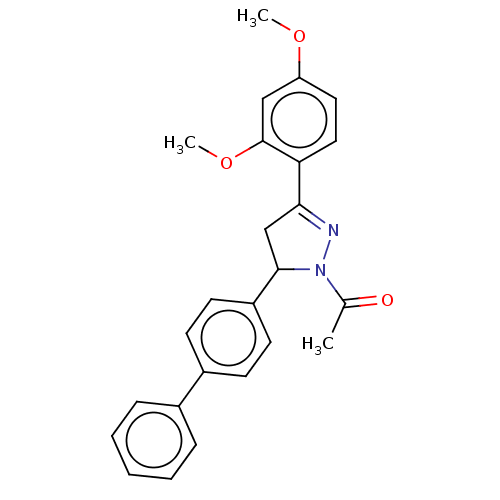
(CHEMBL5183314)Show SMILES COc1ccc(C2=NN(C(C2)c2ccc(cc2)-c2ccccc2)C(C)=O)c(OC)c1 |t:6| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.12E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590179

(CHEMBL5169783)Show SMILES CC(=O)N1N=C(CC1c1ccccc1)c1ccc(cc1)-c1ccccc1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.32E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590185
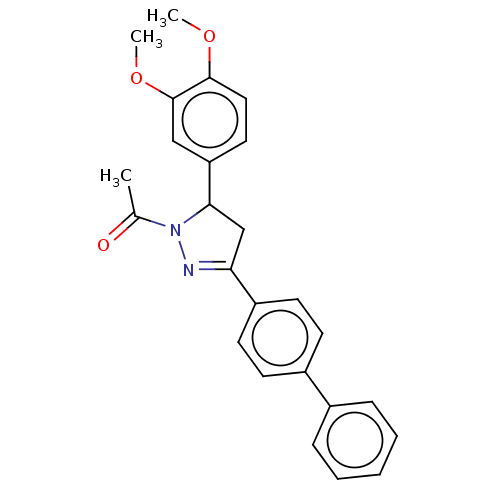
(CHEMBL5169502)Show SMILES COc1ccc(cc1OC)C1CC(=NN1C(C)=O)c1ccc(cc1)-c1ccccc1 |c:13| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.42E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590184

(CHEMBL5208439)Show SMILES COc1ccc(OC)c(c1)C1CC(=NN1C(C)=O)c1ccc(cc1)-c1ccccc1 |c:13| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.42E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590183

(CHEMBL5171946)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)C(O)=O)c1ccc(cc1)-c1ccccc1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.54E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590182
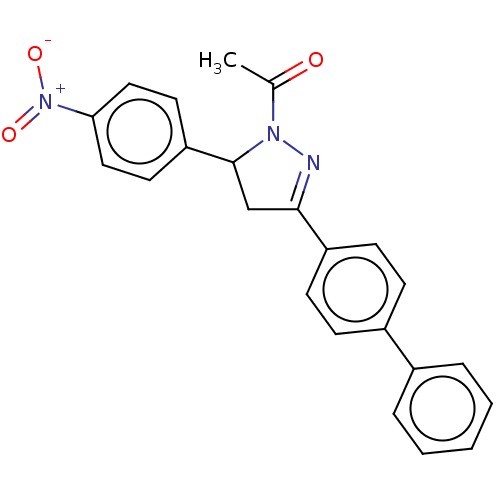
(CHEMBL5182185)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)[N+]([O-])=O)c1ccc(cc1)-c1ccccc1 |c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.55E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590193

(CHEMBL5191224)Show SMILES COc1ccc(OC)c(c1)C1=NN(C(C1)c1ccc(cc1)-c1ccccc1)C(C)=O |t:11| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.72E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50590191
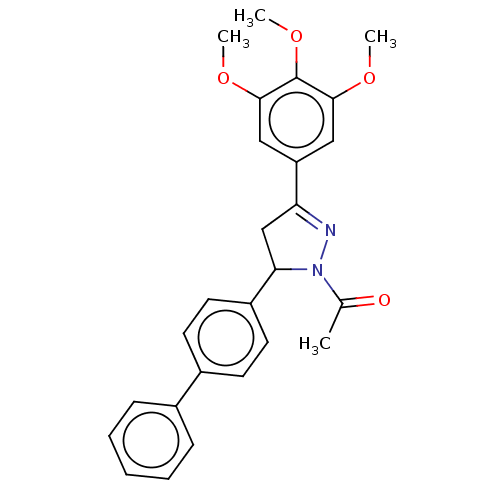
(CHEMBL5184154)Show SMILES COc1cc(cc(OC)c1OC)C1=NN(C(C1)c1ccc(cc1)-c1ccccc1)C(C)=O |t:13| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.94E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00262k
BindingDB Entry DOI: 10.7270/Q2J67MWD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data