

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50018850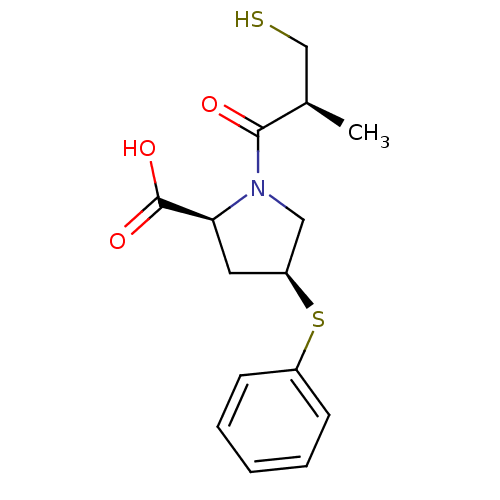 (1-(3-Mercapto-2-methyl-propionyl)-4-phenylsulfanyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory constant against rabbit lung Angiotensin I converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis | J Nat Prod 78: 1671-82 (2015) Article DOI: 10.1021/acs.jnatprod.5b00301 BindingDB Entry DOI: 10.7270/Q22F7Q76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50018849 (4-Cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis | J Nat Prod 78: 1671-82 (2015) Article DOI: 10.1021/acs.jnatprod.5b00301 BindingDB Entry DOI: 10.7270/Q22F7Q76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506773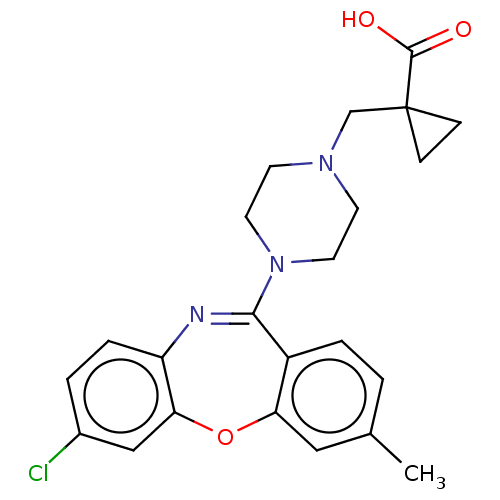 (US11046651, Compound 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506774 (US11046651, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506775 (US11046651, Compound 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506776 (US11046651, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506777 (US11046651, Compound 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506778 (US11046651, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506780 (US11046651, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506781 (US11046651, Compound 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506782 (US11046651, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506784 (US11046651, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506785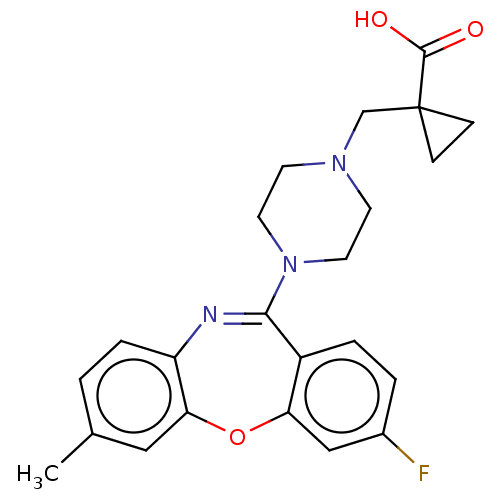 (US11046651, Compound 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506788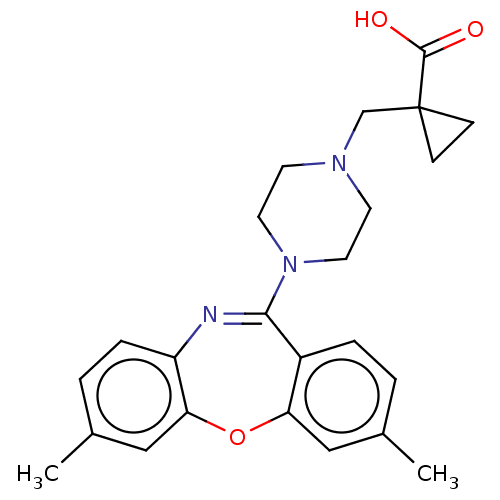 (US11046651, Compound 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506790 (US11046651, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506792 (US11046651, Compound 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506798 (US11046651, Compound 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506799 (US11046651, Compound 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506800 (US11046651, Compound 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506801 (US11046651, Compound 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506793 (US11046651, Compound 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506795 (US11046651, Compound 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506772 (US11046651, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506794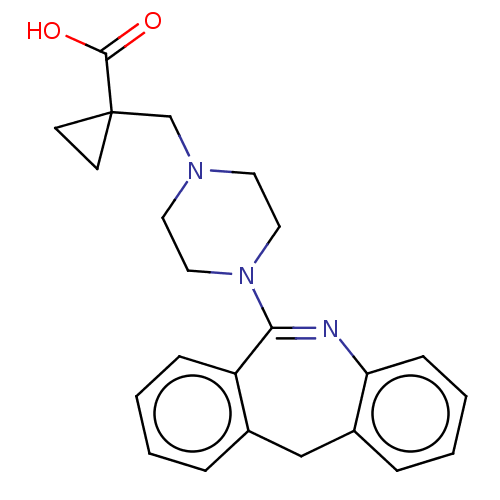 (US11046651, Compound 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506790 (US11046651, Compound 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506783 (US11046651, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506778 (US11046651, Compound 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506781 (US11046651, Compound 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506782 (US11046651, Compound 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506772 (US11046651, Compound 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264192 (CHEMBL4072118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264154 (CHEMBL4100832) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264193 (CHEMBL4084381) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50018848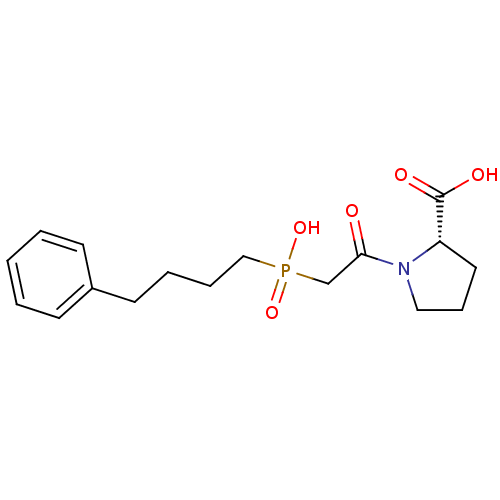 (1-{2-[Hydroxy-(4-phenyl-butyl)-phosphinoyl]-acetyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264185 (CHEMBL4070299) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264192 (CHEMBL4072118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264192 (CHEMBL4072118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264154 (CHEMBL4100832) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264193 (CHEMBL4084381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264184 (CHEMBL4097477) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM41542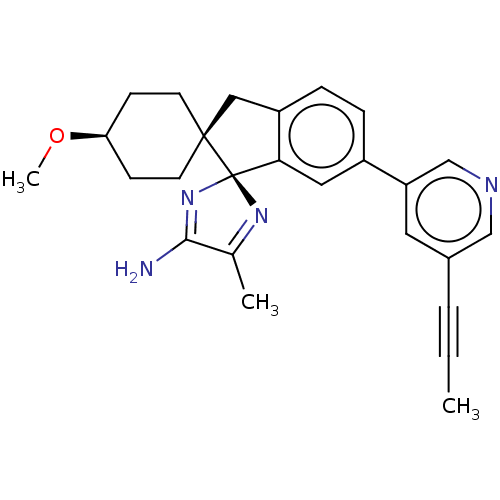 (US8865911, 122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 (1 to 460 residues) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition measu... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296936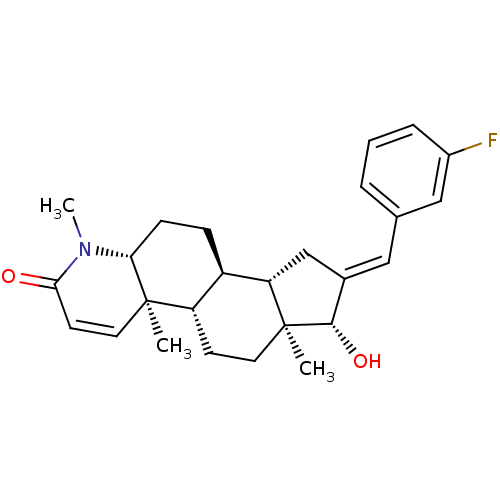 (16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506785 (US11046651, Compound 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506786 (US11046651, Compound 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506783 (US11046651, Compound 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506787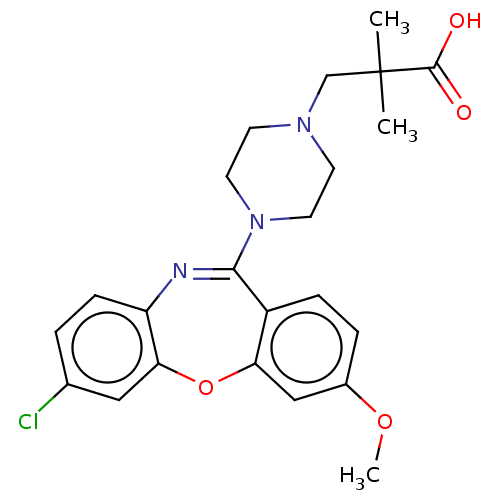 (US11046651, Compound 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2020 total ) | Next | Last >> |