

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50353018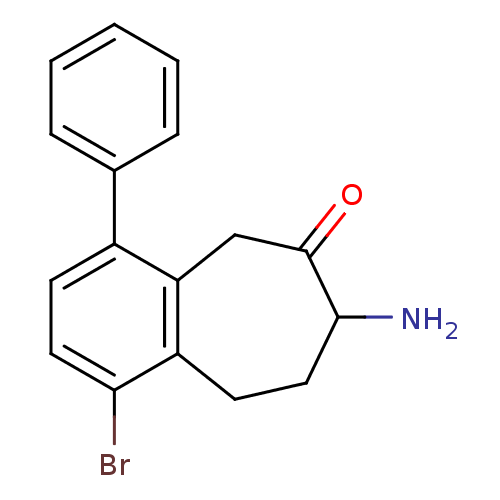 (CHEMBL1821980 | CHEMBL1852660) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute-Alsace Curated by ChEMBL | Assay Description Inhibition of pig APN using L-leucine-p-nitroanilide as substrate measured every 10 mins for 2 hrs by spectriphotometric analysis | Bioorg Med Chem 21: 2135-44 (2013) Article DOI: 10.1016/j.bmc.2012.12.038 BindingDB Entry DOI: 10.7270/Q2CZ38J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Mus musculus) | BDBM50353018 (CHEMBL1821980 | CHEMBL1852660) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute-Alsace Curated by ChEMBL | Assay Description Inhibition of mouse APN using L-leucine-p-nitroanilide as substrate measured every 10 mins for 2 hrs by spectriphotometric analysis | Bioorg Med Chem 21: 2135-44 (2013) Article DOI: 10.1016/j.bmc.2012.12.038 BindingDB Entry DOI: 10.7270/Q2CZ38J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50353018 (CHEMBL1821980 | CHEMBL1852660) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute-Alsace Curated by ChEMBL | Assay Description Inhibition of human APN using L-leucine-p-nitroanilide as substrate measured every 10 mins for 2 hrs by spectriphotometric analysis | Bioorg Med Chem 21: 2135-44 (2013) Article DOI: 10.1016/j.bmc.2012.12.038 BindingDB Entry DOI: 10.7270/Q2CZ38J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50353016 (CHEMBL1821978) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute-Alsace Curated by ChEMBL | Assay Description Inhibition of pig APN using L-leucine-p-nitroanilide as substrate measured every 10 mins for 2 hrs by spectriphotometric analysis | Bioorg Med Chem 21: 2135-44 (2013) Article DOI: 10.1016/j.bmc.2012.12.038 BindingDB Entry DOI: 10.7270/Q2CZ38J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Mus musculus) | BDBM50353016 (CHEMBL1821978) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute-Alsace Curated by ChEMBL | Assay Description Inhibition of mouse APN using L-leucine-p-nitroanilide as substrate measured every 10 mins for 2 hrs by spectriphotometric analysis | Bioorg Med Chem 21: 2135-44 (2013) Article DOI: 10.1016/j.bmc.2012.12.038 BindingDB Entry DOI: 10.7270/Q2CZ38J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50353016 (CHEMBL1821978) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute-Alsace Curated by ChEMBL | Assay Description Inhibition of human APN using L-leucine-p-nitroanilide as substrate measured every 10 mins for 2 hrs by spectriphotometric analysis | Bioorg Med Chem 21: 2135-44 (2013) Article DOI: 10.1016/j.bmc.2012.12.038 BindingDB Entry DOI: 10.7270/Q2CZ38J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50336495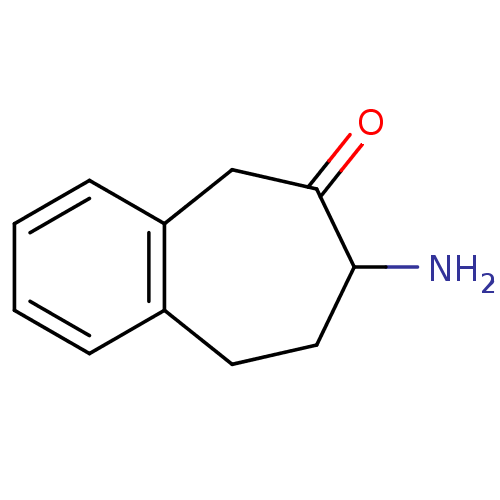 (7-Amino-5,7,8,9-tetrahydro-benzocyclohepten-6-one,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute-Alsace Curated by ChEMBL | Assay Description Inhibition of pig APN using L-leucine-p-nitroanilide as substrate measured every 10 mins for 2 hrs by spectriphotometric analysis | Bioorg Med Chem 21: 2135-44 (2013) Article DOI: 10.1016/j.bmc.2012.12.038 BindingDB Entry DOI: 10.7270/Q2CZ38J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50336495 (7-Amino-5,7,8,9-tetrahydro-benzocyclohepten-6-one,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute-Alsace Curated by ChEMBL | Assay Description Inhibition of human APN using L-leucine-p-nitroanilide as substrate measured every 10 mins for 2 hrs by spectriphotometric analysis | Bioorg Med Chem 21: 2135-44 (2013) Article DOI: 10.1016/j.bmc.2012.12.038 BindingDB Entry DOI: 10.7270/Q2CZ38J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Mus musculus) | BDBM50336495 (7-Amino-5,7,8,9-tetrahydro-benzocyclohepten-6-one,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute-Alsace Curated by ChEMBL | Assay Description Inhibition of mouse APN using L-leucine-p-nitroanilide as substrate measured every 10 mins for 2 hrs by spectriphotometric analysis | Bioorg Med Chem 21: 2135-44 (2013) Article DOI: 10.1016/j.bmc.2012.12.038 BindingDB Entry DOI: 10.7270/Q2CZ38J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Staphylococcus aureus) | BDBM141970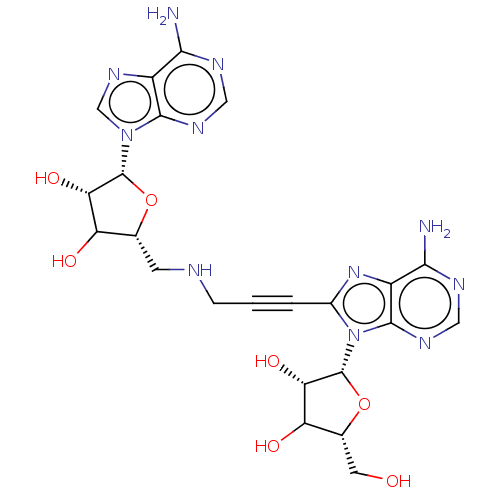 (US8927520, 19) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur; Institut Curie US Patent | Assay Description For inhibitor assays, IC50 was determined, in the presence of 1 mM NAD and 4 mM ATP (for LmNADK1) or 2 mM ATP (for SaNADK). Dixon plots were used to ... | US Patent US8927520 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6QX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase 1 (Listeria monocytogenes) | BDBM141970 (US8927520, 19) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur; Institut Curie US Patent | Assay Description For inhibitor assays, IC50 was determined, in the presence of 1 mM NAD and 4 mM ATP (for LmNADK1) or 2 mM ATP (for SaNADK). Dixon plots were used to ... | US Patent US8927520 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6QX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Staphylococcus aureus) | BDBM141971 (US8927520, 22) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur; Institut Curie US Patent | Assay Description For inhibitor assays, IC50 was determined, in the presence of 1 mM NAD and 4 mM ATP (for LmNADK1) or 2 mM ATP (for SaNADK). Dixon plots were used to ... | US Patent US8927520 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6QX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase 1 (Listeria monocytogenes) | BDBM141971 (US8927520, 22) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur; Institut Curie US Patent | Assay Description For inhibitor assays, IC50 was determined, in the presence of 1 mM NAD and 4 mM ATP (for LmNADK1) or 2 mM ATP (for SaNADK). Dixon plots were used to ... | US Patent US8927520 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6QX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208878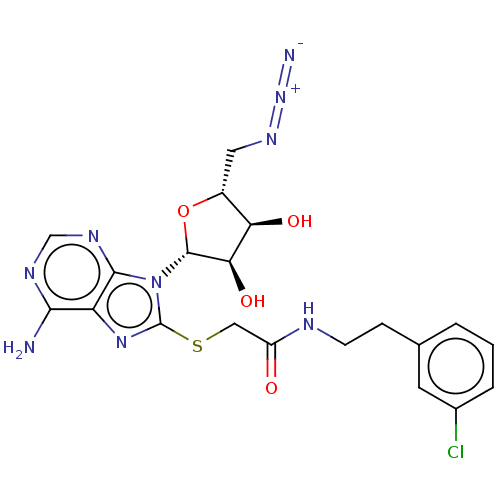 (CHEMBL3883415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase 1 (Listeria monocytogenes) | BDBM141969 (US8927520, 17) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur; Institut Curie US Patent | Assay Description For inhibitor assays, IC50 was determined, in the presence of 1 mM NAD and 4 mM ATP (for LmNADK1) or 2 mM ATP (for SaNADK). Dixon plots were used to ... | US Patent US8927520 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6QX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Staphylococcus aureus) | BDBM141969 (US8927520, 17) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur; Institut Curie US Patent | Assay Description For inhibitor assays, IC50 was determined, in the presence of 1 mM NAD and 4 mM ATP (for LmNADK1) or 2 mM ATP (for SaNADK). Dixon plots were used to ... | US Patent US8927520 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6QX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208780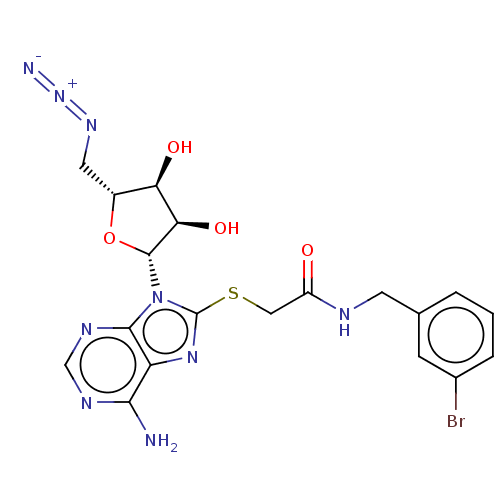 (CHEMBL3884180) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208778 (CHEMBL3885397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208802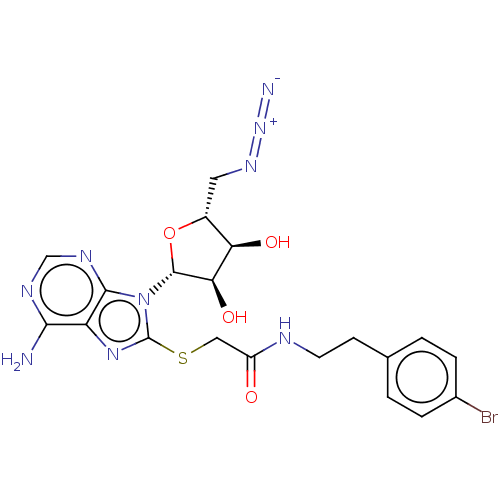 (CHEMBL3885465) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208739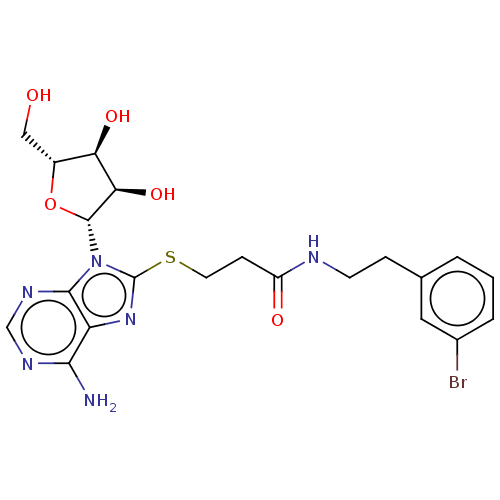 (CHEMBL3885207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208736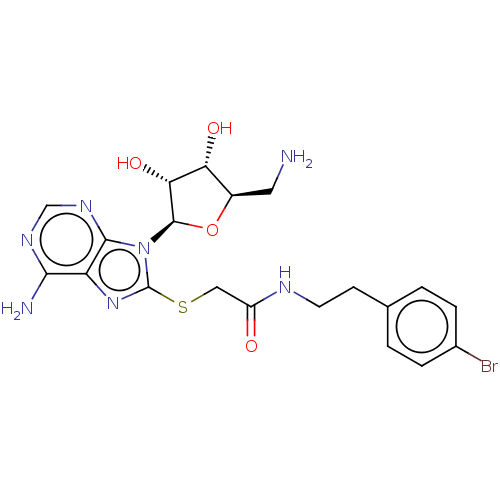 (CHEMBL3883373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208741 (CHEMBL3883614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208904 (CHEMBL3884876) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208740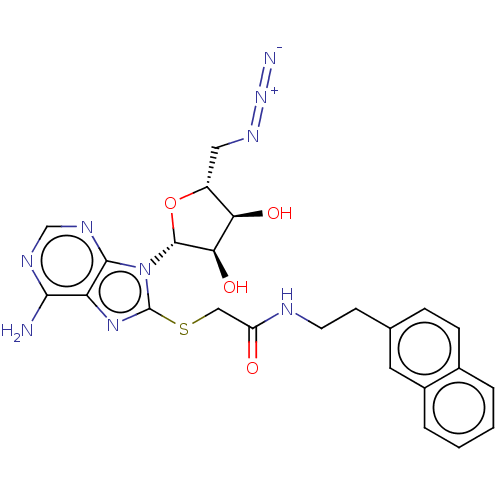 (CHEMBL3885345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208805 (CHEMBL3883397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208745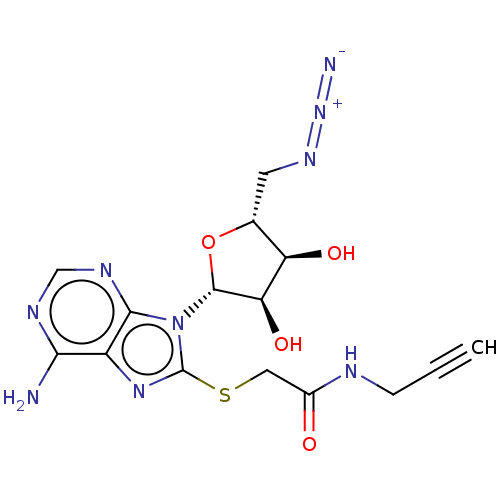 (CHEMBL3884239) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208743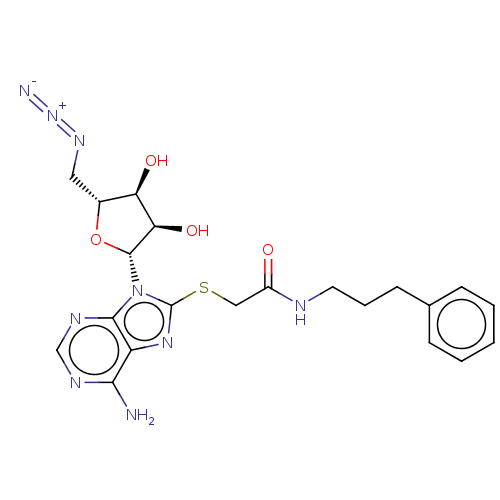 (CHEMBL3884081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208737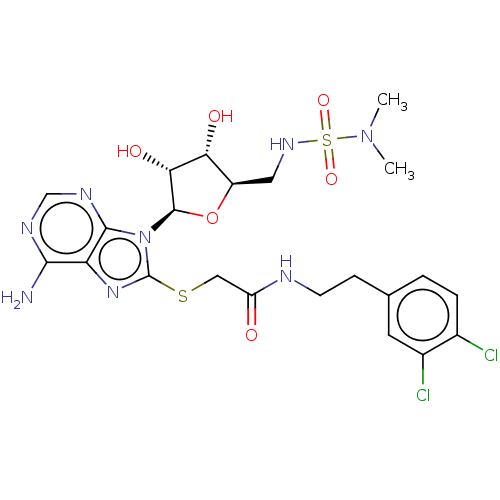 (CHEMBL3884746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208779 (CHEMBL3884775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208795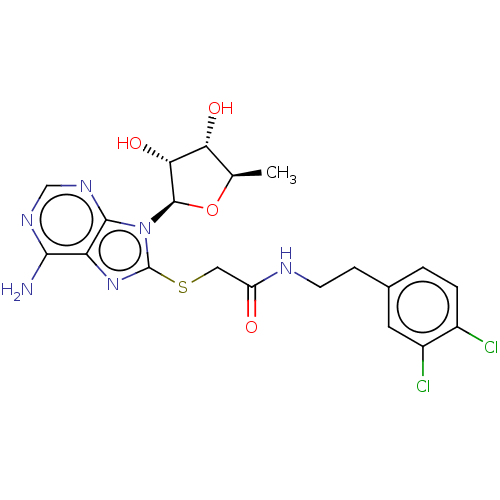 (CHEMBL3885356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208735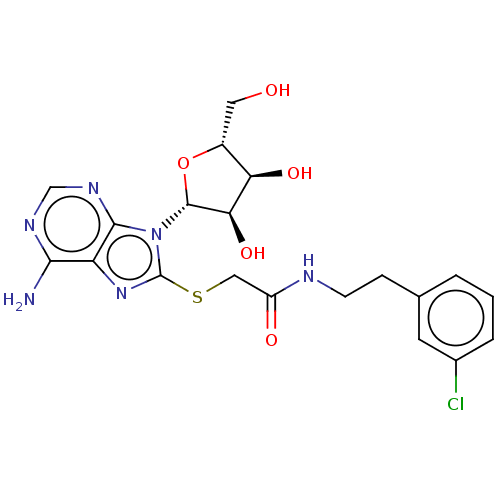 (CHEMBL3884857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208774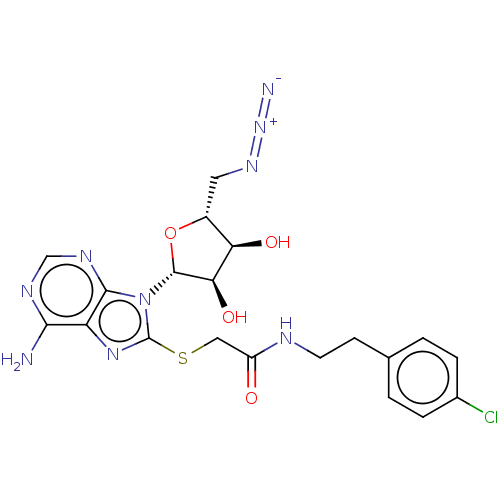 (CHEMBL3885310) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208874 (CHEMBL3883624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208781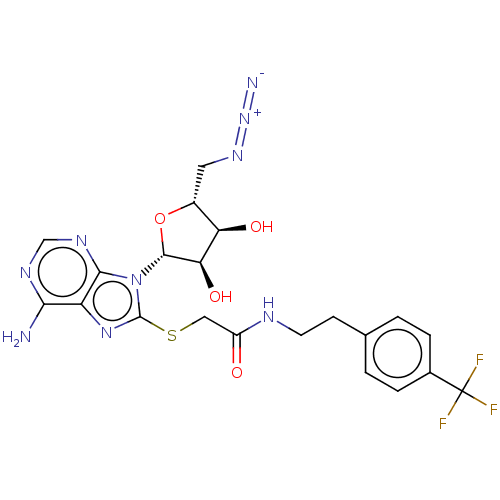 (CHEMBL3883763) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208773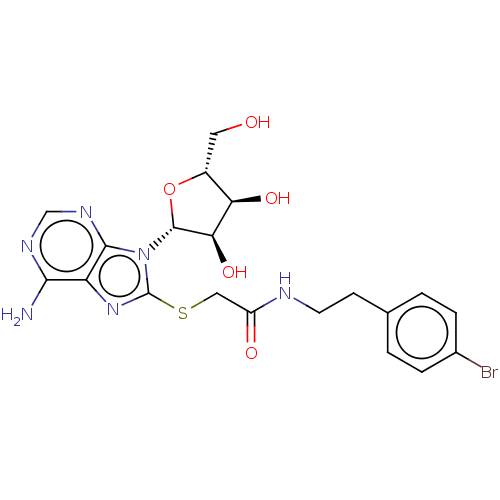 (CHEMBL3884901) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208775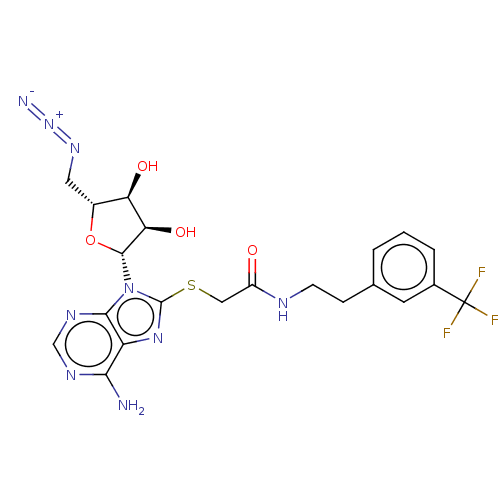 (CHEMBL3884119) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208742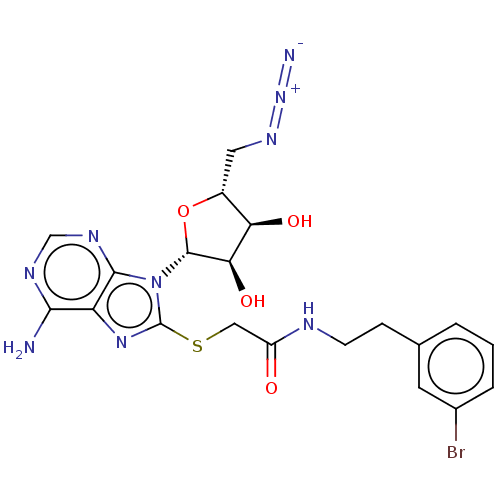 (CHEMBL3884982) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208734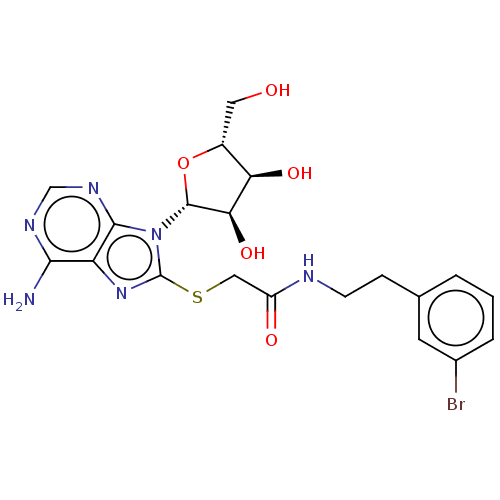 (CHEMBL3883980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 3.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208738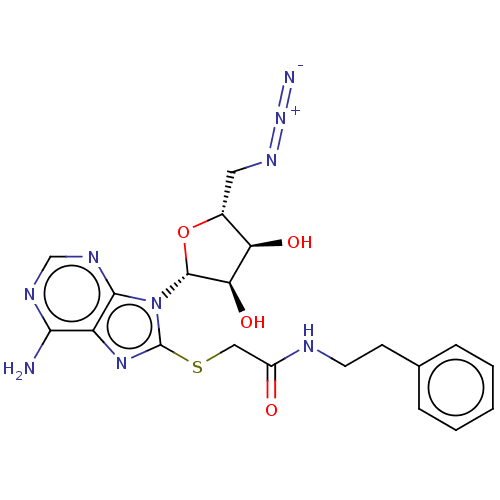 (CHEMBL3885227) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208744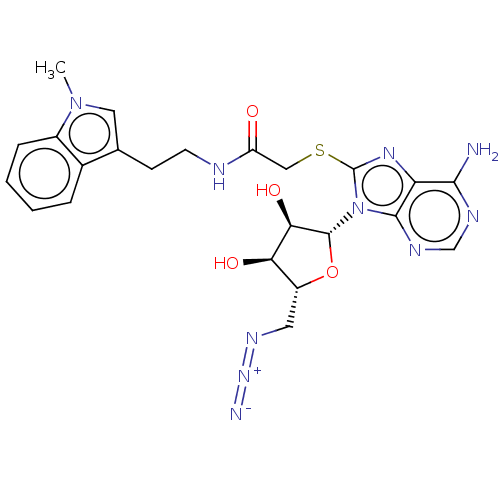 (CHEMBL3885137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208776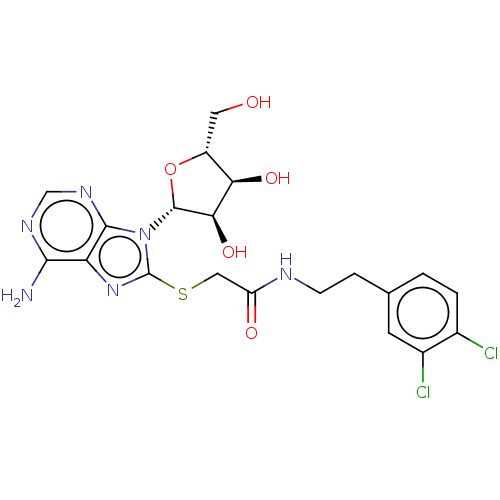 (CHEMBL3883970) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208777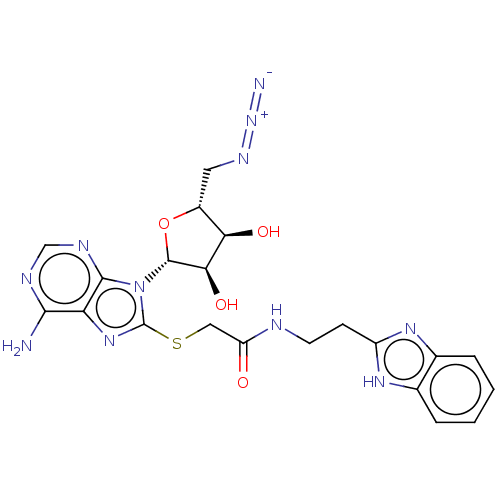 (CHEMBL3883775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 5.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD kinase (Homo sapiens (Human)) | BDBM50208905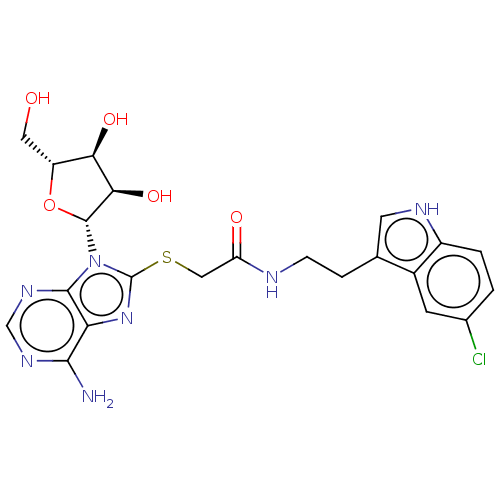 (CHEMBL3884652) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; CNRS Curated by ChEMBL | Assay Description Inhibition of human NADK expressed in Escherichia coli BL21(DE3) assessed as reduction in NADP production using NAD as substrate by glucose-6-phospha... | Eur J Med Chem 124: 1041-1056 (2016) Article DOI: 10.1016/j.ejmech.2016.10.033 BindingDB Entry DOI: 10.7270/Q2WH2S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50428286 (DABRAFENIB | GSK2118436A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01354 BindingDB Entry DOI: 10.7270/Q2474FZQ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50602851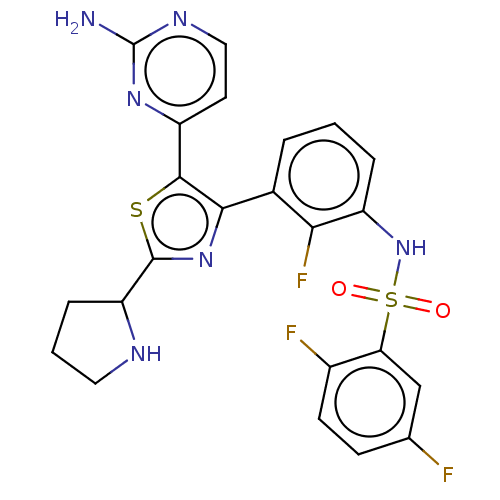 (CHEMBL5189539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01354 BindingDB Entry DOI: 10.7270/Q2474FZQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50602852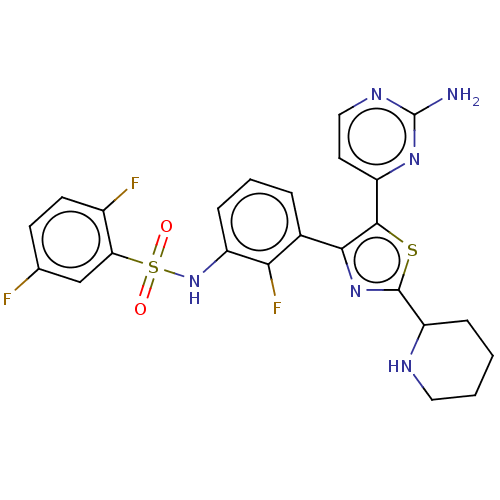 (CHEMBL5201169) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01354 BindingDB Entry DOI: 10.7270/Q2474FZQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50602855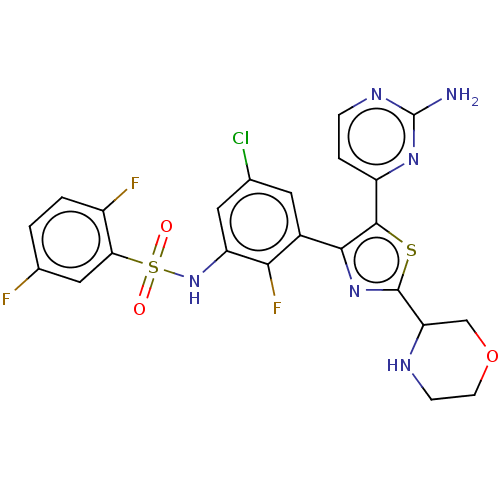 (CHEMBL5176494) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01354 BindingDB Entry DOI: 10.7270/Q2474FZQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50602850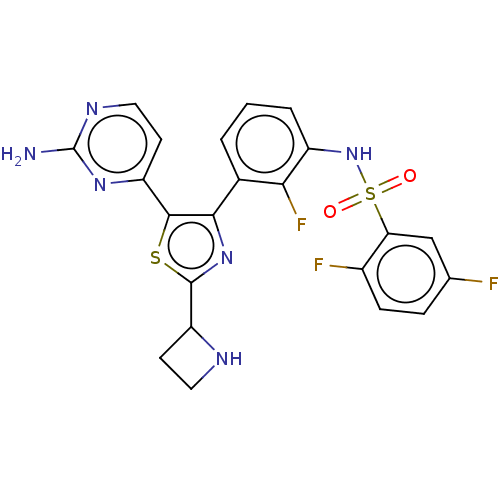 (CHEMBL5203353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01354 BindingDB Entry DOI: 10.7270/Q2474FZQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50602854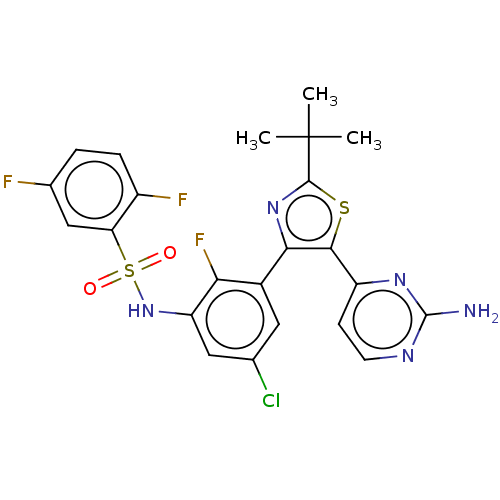 (CHEMBL5202550) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01354 BindingDB Entry DOI: 10.7270/Q2474FZQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50602856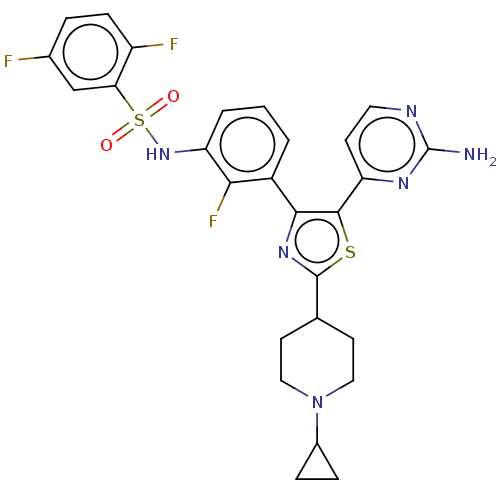 (CHEMBL5183233) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01354 BindingDB Entry DOI: 10.7270/Q2474FZQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 305 total ) | Next | Last >> |