 Found 452 hits with Last Name = 'glass' and Initial = 'm'
Found 452 hits with Last Name = 'glass' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50404173

(CHEMBL5266788)Show SMILES COc1ccc(CN(CCN(C)CCCCCCCCNC(=O)c2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c2ccccn2)cc1 |(-6.44,-11.63,;-6.45,-10.08,;-5.11,-9.25,;-3.76,-9.97,;-2.44,-9.15,;-2.44,-7.6,;-1.12,-6.76,;-1.13,-5.22,;.21,-4.38,;1.54,-5.11,;2.87,-4.27,;2.87,-2.71,;4.22,-4.99,;5.53,-4.19,;6.88,-4.92,;8.18,-4.11,;9.55,-4.85,;10.85,-4.04,;12.2,-4.77,;13.51,-3.97,;14.86,-4.69,;16.17,-3.87,;17.52,-4.64,;16.14,-2.29,;14.81,-1.54,;14.78,.02,;16.1,.84,;17.42,.09,;17.45,-1.49,;18.89,.5,;19.98,-.61,;19.35,1.53,;16.06,2.44,;14.75,3.18,;13.42,2.37,;12.08,3.14,;12.07,4.74,;10.72,5.49,;13.39,5.53,;14.72,4.76,;16.03,5.56,;17.37,4.81,;18.66,5.6,;19.98,4.83,;21.31,5.6,;20,3.26,;18.7,2.46,;17.38,3.23,;-2.48,-4.48,;-3.81,-5.32,;-5.16,-4.62,;-5.18,-3.05,;-3.84,-2.21,;-2.51,-2.94,;-3.79,-6.88,;-5.14,-7.69,)| Show InChI InChI=1S/C45H48N4O7/c1-48(25-26-49(42-11-7-9-22-46-42)30-31-12-17-35(55-2)18-13-31)24-10-6-4-3-5-8-23-47-44(52)32-14-19-36(39(27-32)45(53)54)43-37-20-15-33(50)28-40(37)56-41-29-34(51)16-21-38(41)43/h7,9,11-22,27-29,50H,3-6,8,10,23-26,30H2,1-2H3,(H,47,52)(H,53,54) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro antagonism of the 5-HT-3 receptor determined by inhibition of 5-HT-induced depolarization of the isolated rat vagus nerve. |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590329
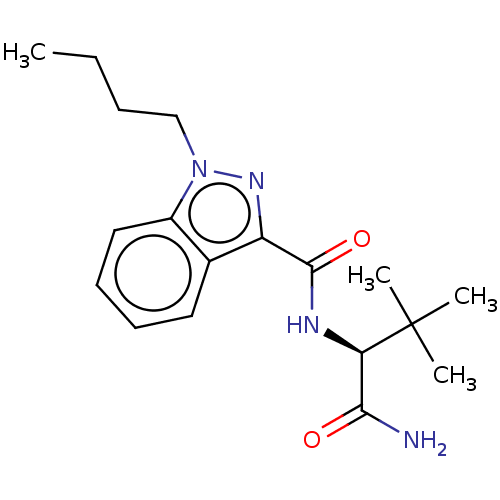
(CHEMBL5169682)Show SMILES CCCCn1nc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590329

(CHEMBL5169682)Show SMILES CCCCn1nc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50404172

(CHEMBL5273987)Show SMILES COc1ccc(CN(CCN(C)CCCCCCNS(=O)(=O)c2cccc3c(cccc23)N(C)C)c2ccccn2)cc1 Show InChI InChI=1S/C34H45N5O3S/c1-37(2)32-15-11-14-31-30(32)13-12-16-33(31)43(40,41)36-23-8-5-6-10-24-38(3)25-26-39(34-17-7-9-22-35-34)27-28-18-20-29(42-4)21-19-28/h7,9,11-22,36H,5-6,8,10,23-27H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro antagonism of the 5-HT-3 receptor determined by inhibition of 5-HT-induced depolarization of the isolated rat vagus nerve. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517230

(CHEMBL4467984)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 |r| Show InChI InChI=1S/C64H83Cl3N10O8/c1-5-38-76(51-28-29-52-47(40-51)15-14-18-57(52)80)39-33-45-19-26-50(27-20-45)74-59(82)32-31-58(81)68-34-12-8-16-54(72-43(3)78)63(84)70-37-13-9-17-55(73-44(4)79)62(83)69-35-10-6-7-11-36-71-64(85)60-42(2)61(46-21-23-48(65)24-22-46)77(75-60)56-30-25-49(66)41-53(56)67/h14-15,18-27,30,41,51,54-55,80H,5-13,16-17,28-29,31-40H2,1-4H3,(H,68,81)(H,69,83)(H,70,84)(H,71,85)(H,72,78)(H,73,79)(H,74,82)/t51?,54-,55-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590329

(CHEMBL5169682)Show SMILES CCCCn1nc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590329

(CHEMBL5169682)Show SMILES CCCCn1nc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517232
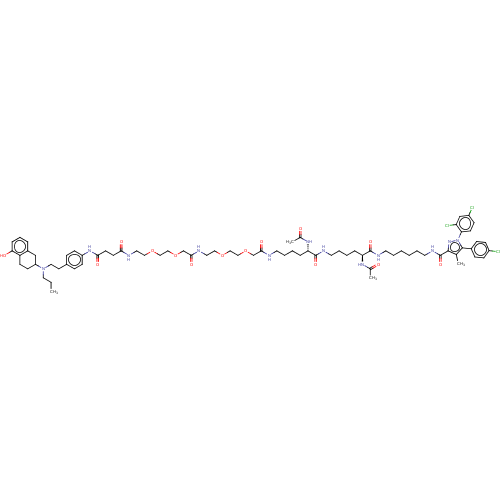
(CHEMBL4546839)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 |r| Show InChI InChI=1S/C76H105Cl3N12O14/c1-5-40-90(61-28-29-62-57(48-61)15-14-18-67(62)94)41-33-55-19-26-60(27-20-55)88-69(96)32-31-68(95)81-38-42-102-44-47-105-51-71(98)82-39-43-103-45-46-104-50-70(97)80-34-12-8-16-64(86-53(3)92)75(100)84-37-13-9-17-65(87-54(4)93)74(99)83-35-10-6-7-11-36-85-76(101)72-52(2)73(56-21-23-58(77)24-22-56)91(89-72)66-30-25-59(78)49-63(66)79/h14-15,18-27,30,49,61,64-65,94H,5-13,16-17,28-29,31-48,50-51H2,1-4H3,(H,80,97)(H,81,95)(H,82,98)(H,83,99)(H,84,100)(H,85,101)(H,86,92)(H,87,93)(H,88,96)/t61?,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590328

(CHEMBL5180182)Show SMILES CCCCn1nc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590328

(CHEMBL5180182)Show SMILES CCCCn1nc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590326

(CHEMBL5202843)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590326

(CHEMBL5202843)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590326

(CHEMBL5202843)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590326

(CHEMBL5202843)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517229
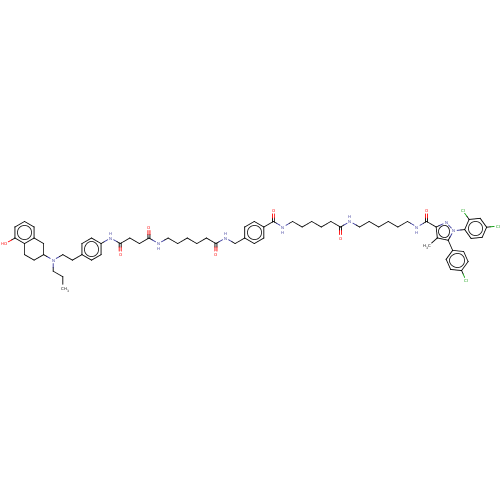
(CHEMBL4541515)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCCCCC(=O)NCc2ccc(cc2)C(=O)NCCCCCC(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 Show InChI InChI=1S/C68H84Cl3N9O7/c1-3-42-79(56-32-33-57-52(44-56)15-14-16-60(57)81)43-37-48-21-30-55(31-22-48)77-64(85)36-35-63(84)73-39-12-6-9-18-62(83)76-46-49-19-23-51(24-20-49)67(86)74-40-13-7-8-17-61(82)72-38-10-4-5-11-41-75-68(87)65-47(2)66(50-25-27-53(69)28-26-50)80(78-65)59-34-29-54(70)45-58(59)71/h14-16,19-31,34,45,56,81H,3-13,17-18,32-33,35-44,46H2,1-2H3,(H,72,82)(H,73,84)(H,74,86)(H,75,87)(H,76,83)(H,77,85) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517236

(CHEMBL4465127)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCOCCOCC(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 |r| Show InChI InChI=1S/C70H94Cl3N11O11/c1-5-39-83(56-28-29-57-52(44-56)15-14-18-62(57)87)40-33-50-19-26-55(27-20-50)81-64(89)32-31-63(88)75-38-41-94-42-43-95-46-65(90)74-34-12-8-16-59(79-48(3)85)69(92)77-37-13-9-17-60(80-49(4)86)68(91)76-35-10-6-7-11-36-78-70(93)66-47(2)67(51-21-23-53(71)24-22-51)84(82-66)61-30-25-54(72)45-58(61)73/h14-15,18-27,30,45,56,59-60,87H,5-13,16-17,28-29,31-44,46H2,1-4H3,(H,74,90)(H,75,88)(H,76,91)(H,77,92)(H,78,93)(H,79,85)(H,80,86)(H,81,89)/t56?,59-,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50515386
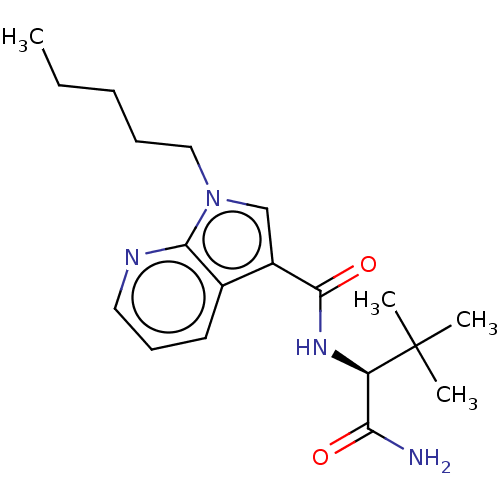
(CHEMBL4476642)Show SMILES CCCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2cccnc12 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-6-7-11-23-12-14(13-9-8-10-21-17(13)23)18(25)22-15(16(20)24)19(2,3)4/h8-10,12,15H,5-7,11H2,1-4H3,(H2,20,24)(H,22,25)/t15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50515386

(CHEMBL4476642)Show SMILES CCCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2cccnc12 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-6-7-11-23-12-14(13-9-8-10-21-17(13)23)18(25)22-15(16(20)24)19(2,3)4/h8-10,12,15H,5-7,11H2,1-4H3,(H2,20,24)(H,22,25)/t15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517222
(CHEMBL4579585)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCCCCC(=O)NCc2ccc(cc2)C(=O)NCCCCCC(=O)NCCCCCC(=O)NCc2ccc(cc2)C(=O)NCCCCCC(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 Show InChI InChI=1S/C88H113Cl3N12O10/c1-3-56-102(73-44-45-74-69(58-73)21-20-22-77(74)104)57-49-63-31-42-72(43-32-63)100-83(110)48-47-82(109)94-52-17-7-13-26-81(108)99-61-65-29-35-68(36-30-65)87(112)96-54-19-9-11-24-79(106)93-51-16-6-12-25-80(107)98-60-64-27-33-67(34-28-64)86(111)95-53-18-8-10-23-78(105)92-50-14-4-5-15-55-97-88(113)84-62(2)85(66-37-39-70(89)40-38-66)103(101-84)76-46-41-71(90)59-75(76)91/h20-22,27-43,46,59,73,104H,3-19,23-26,44-45,47-58,60-61H2,1-2H3,(H,92,105)(H,93,106)(H,94,109)(H,95,111)(H,96,112)(H,97,113)(H,98,107)(H,99,108)(H,100,110) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50465034

(CHEMBL4294558)Show SMILES CCCOc1cccc2c(cn(CC3CCOCC3)c12)C(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C25H29NO4/c1-3-13-30-23-6-4-5-21-22(25(27)19-7-9-20(28-2)10-8-19)17-26(24(21)23)16-18-11-14-29-15-12-18/h4-10,17-18H,3,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Otago
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from N-terminal HA-tagged human CB2 receptor expressed in HEK293 cell membranes after 1 hr by microbeta scintillation co... |
Eur J Med Chem 145: 770-789 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.076
BindingDB Entry DOI: 10.7270/Q2X069QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590328

(CHEMBL5180182)Show SMILES CCCCn1nc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50590328

(CHEMBL5180182)Show SMILES CCCCn1nc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21279
(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517233

(CHEMBL4568756)Show SMILES CCOC(=O)c1ccc(CNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C27H22Cl3N3O3/c1-3-36-27(35)19-6-4-17(5-7-19)15-31-26(34)24-16(2)25(18-8-10-20(28)11-9-18)33(32-24)23-13-12-21(29)14-22(23)30/h4-14H,3,15H2,1-2H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517221
(CHEMBL4554135)Show SMILES CC(=O)OCCC#Cc1ccc(cc1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C28H28Cl2N4O3/c1-19-26(28(36)32-33-15-5-3-6-16-33)31-34(25-14-13-23(29)18-24(25)30)27(19)22-11-9-21(10-12-22)8-4-7-17-37-20(2)35/h9-14,18H,3,5-7,15-17H2,1-2H3,(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590325
(CHEMBL5208741)Show SMILES CCCCn1cc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590325

(CHEMBL5208741)Show SMILES CCCCn1cc(C(=O)N[C@@H](C(C)C)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro antagonism of the 5-HT-3 receptor determined by inhibition of 5-HT-induced depolarization of the isolated rat vagus nerve. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50010289
((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C21H27NO/c1-2-14-22(15-13-17-7-4-3-5-8-17)19-11-12-20-18(16-19)9-6-10-21(20)23/h3-10,19,23H,2,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H] spiperone from human D2 dopamine receptor expressed in monkey caudate-putamen membranes |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50404173

(CHEMBL5266788)Show SMILES COc1ccc(CN(CCN(C)CCCCCCCCNC(=O)c2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c2ccccn2)cc1 |(-6.44,-11.63,;-6.45,-10.08,;-5.11,-9.25,;-3.76,-9.97,;-2.44,-9.15,;-2.44,-7.6,;-1.12,-6.76,;-1.13,-5.22,;.21,-4.38,;1.54,-5.11,;2.87,-4.27,;2.87,-2.71,;4.22,-4.99,;5.53,-4.19,;6.88,-4.92,;8.18,-4.11,;9.55,-4.85,;10.85,-4.04,;12.2,-4.77,;13.51,-3.97,;14.86,-4.69,;16.17,-3.87,;17.52,-4.64,;16.14,-2.29,;14.81,-1.54,;14.78,.02,;16.1,.84,;17.42,.09,;17.45,-1.49,;18.89,.5,;19.98,-.61,;19.35,1.53,;16.06,2.44,;14.75,3.18,;13.42,2.37,;12.08,3.14,;12.07,4.74,;10.72,5.49,;13.39,5.53,;14.72,4.76,;16.03,5.56,;17.37,4.81,;18.66,5.6,;19.98,4.83,;21.31,5.6,;20,3.26,;18.7,2.46,;17.38,3.23,;-2.48,-4.48,;-3.81,-5.32,;-5.16,-4.62,;-5.18,-3.05,;-3.84,-2.21,;-2.51,-2.94,;-3.79,-6.88,;-5.14,-7.69,)| Show InChI InChI=1S/C45H48N4O7/c1-48(25-26-49(42-11-7-9-22-46-42)30-31-12-17-35(55-2)18-13-31)24-10-6-4-3-5-8-23-47-44(52)32-14-19-36(39(27-32)45(53)54)43-37-20-15-33(50)28-40(37)56-41-29-34(51)16-21-38(41)43/h7,9,11-22,27-29,50H,3-6,8,10,23-26,30H2,1-2H3,(H,47,52)(H,53,54) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro antagonism of the 5-HT-3 receptor determined by inhibition of 5-HT-induced depolarization of the isolated rat vagus nerve. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517223
(CHEMBL4471116)Show SMILES COC(=O)CCCCCNC(=O)c1nn(c(c1C)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H24Cl3N3O3/c1-15-22(24(32)28-13-5-3-4-6-21(31)33-2)29-30(20-12-11-18(26)14-19(20)27)23(15)16-7-9-17(25)10-8-16/h7-12,14H,3-6,13H2,1-2H3,(H,28,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200170

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)N[C@H]2[C@@]3(C)CC[C@H](C3)C2(C)C)cc1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Otago
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human CB2 receptor |
Eur J Med Chem 145: 770-789 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.076
BindingDB Entry DOI: 10.7270/Q2X069QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200170

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)N[C@H]2[C@@]3(C)CC[C@H](C3)C2(C)C)cc1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Otago
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human CB2 receptor |
Eur J Med Chem 145: 770-789 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.076
BindingDB Entry DOI: 10.7270/Q2X069QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590332
(CHEMBL5181997)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2cccnc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590332

(CHEMBL5181997)Show SMILES CCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2cccnc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50515386

(CHEMBL4476642)Show SMILES CCCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2cccnc12 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-6-7-11-23-12-14(13-9-8-10-21-17(13)23)18(25)22-15(16(20)24)19(2,3)4/h8-10,12,15H,5-7,11H2,1-4H3,(H2,20,24)(H,22,25)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50515386

(CHEMBL4476642)Show SMILES CCCCCn1cc(C(=O)N[C@H](C(N)=O)C(C)(C)C)c2cccnc12 |r| Show InChI InChI=1S/C19H28N4O2/c1-5-6-7-11-23-12-14(13-9-8-10-21-17(13)23)18(25)22-15(16(20)24)19(2,3)4/h8-10,12,15H,5-7,11H2,1-4H3,(H2,20,24)(H,22,25)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50110953
(4-Methyl-1-pentyl-5-phenyl-1H-pyrazole-3-carboxyli...)Show InChI InChI=1S/C21H30N4O/c1-3-4-9-16-25-20(18-12-7-5-8-13-18)17(2)19(22-25)21(26)23-24-14-10-6-11-15-24/h5,7-8,12-13H,3-4,6,9-11,14-16H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro antagonism of the 5-HT-3 receptor determined by inhibition of 5-HT-induced depolarization of the isolated rat vagus nerve. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50399157
(CHEMBL2179724)Show SMILES CCCCCCC(C)(C)c1cc(OC)c([C@H]2C=C(CO)[C@H]3C[C@@H]2C3(C)C)c(OC)c1 |r,t:16| Show InChI InChI=1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro antagonism of the 5-HT-3 receptor determined by inhibition of 5-HT-induced depolarization of the isolated rat vagus nerve. |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590330
(CHEMBL5184576)Show SMILES CCCCn1nc(C(=O)N[C@@H](Cc2ccccc2)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50590330

(CHEMBL5184576)Show SMILES CCCCn1nc(C(=O)N[C@@H](Cc2ccccc2)C(N)=O)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00242b
BindingDB Entry DOI: 10.7270/Q2G73JQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160434
((6-iodo-2-methyl-1-(2-morpholinoethyl)-1H-indol-3-...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(CCN2CCOCC2)c2cc(I)ccc12 Show InChI InChI=1S/C23H25IN2O3/c1-16-22(23(27)17-3-6-19(28-2)7-4-17)20-8-5-18(24)15-21(20)26(16)10-9-25-11-13-29-14-12-25/h3-8,15H,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Otago
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human CB2 receptor |
Eur J Med Chem 145: 770-789 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.076
BindingDB Entry DOI: 10.7270/Q2X069QD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data