

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16694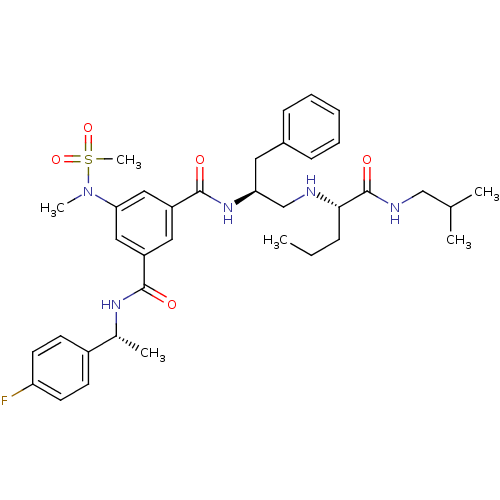 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-5-(N-methylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16693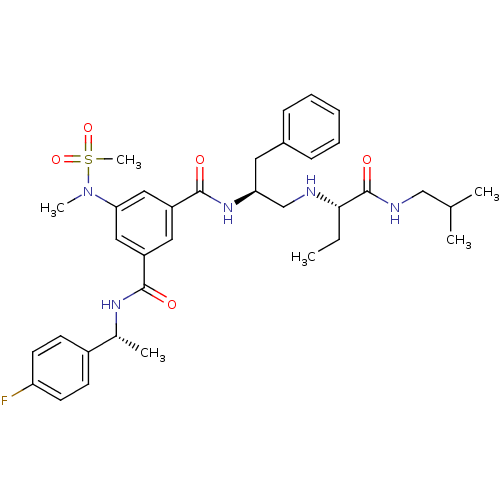 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-5-(N-methylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16692 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-5-(N-methylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16695 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-3-N-[(2S)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16696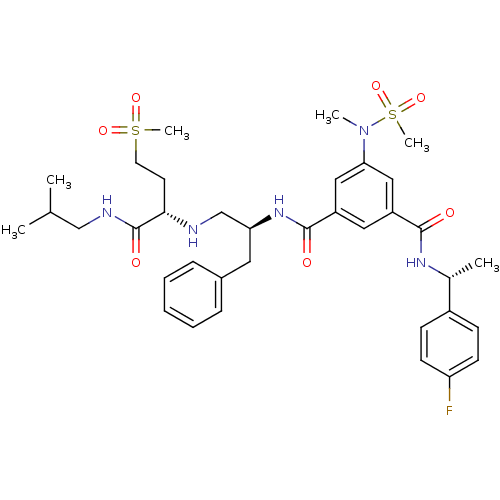 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-3-N-[(2S)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16701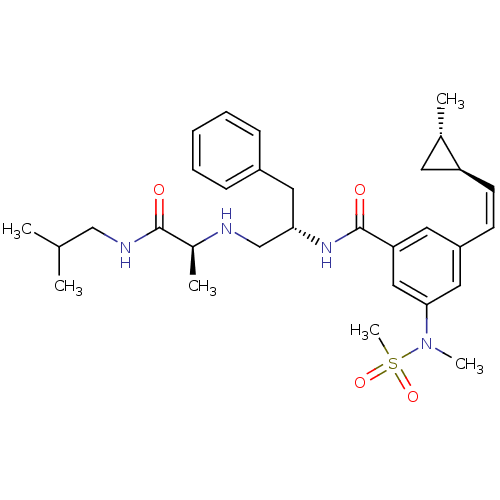 ((2S)-2-{[(2S)-2-({3-[(Z)-2-[(1S,2S)-2-methylcyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16698 (1-N-[(2S)-1-{[(1S)-1-(cyclopropylcarbamoyl)ethyl]a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360722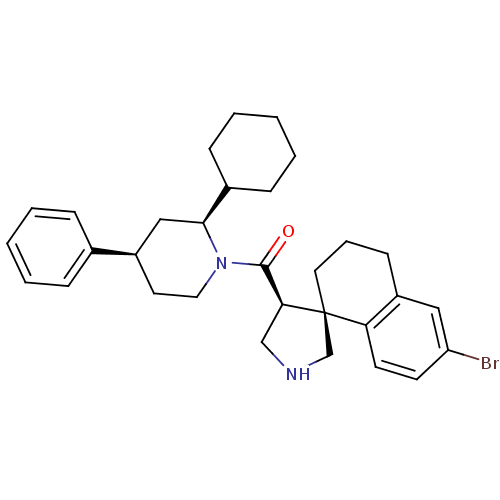 (CHEMBL1934294) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360723 (CHEMBL1934295) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50360723 (CHEMBL1934295) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE2 | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360724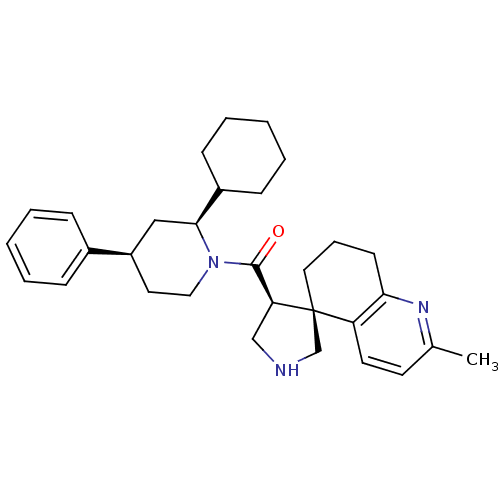 (CHEMBL1934296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16697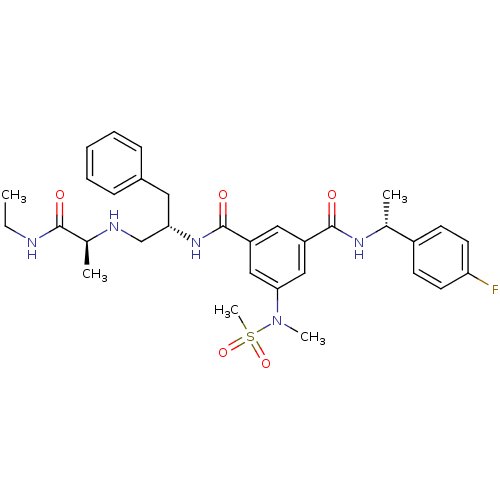 (1-N-[(2S)-1-{[(1S)-1-(ethylcarbamoyl)ethyl]amino}-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360721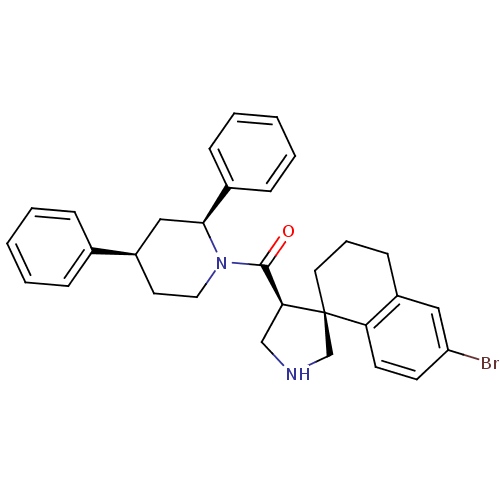 (CHEMBL1934293) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360724 (CHEMBL1934296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in mouse fibroblast cells assessed as inhibition of soluble APPbeta_NF cleavage | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16712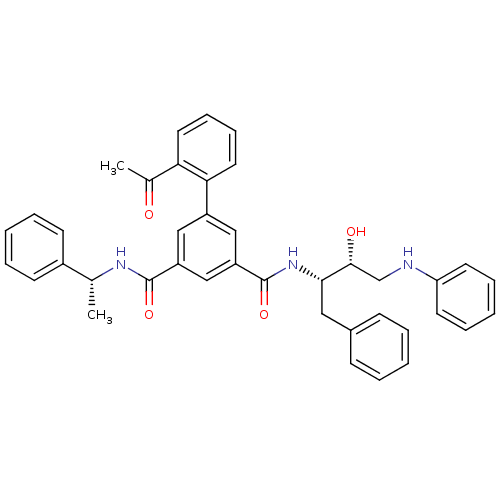 (5-(2-acetylphenyl)-1-N-[(2S,3R)-3-hydroxy-1-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16711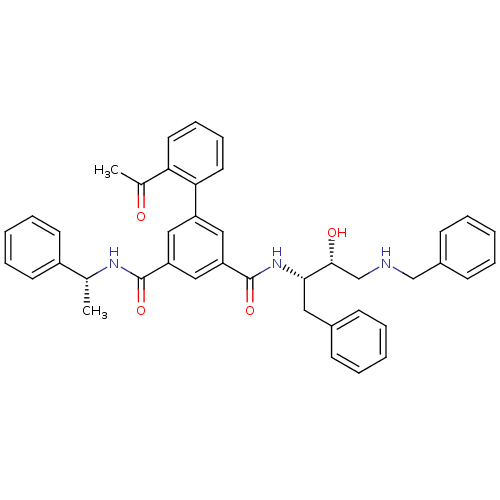 (1-N-[(2S,3R)-4-(benzylamino)-3-hydroxy-1-phenylbut...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16713 (5-(2-acetylphenyl)-1-N-[(2S,3R)-3-hydroxy-1-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16691 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-5-(N-methylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16699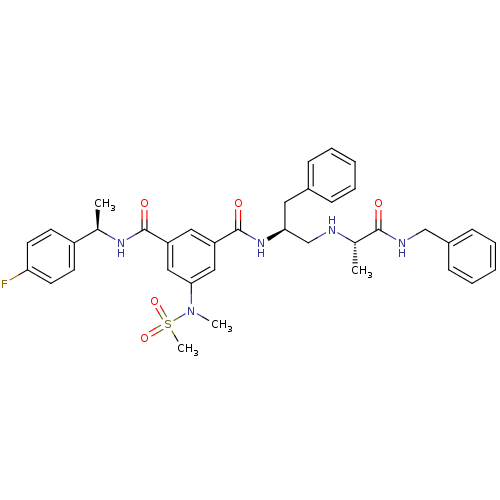 (1-N-[(2S)-1-{[(1S)-1-(benzylcarbamoyl)ethyl]amino}...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360720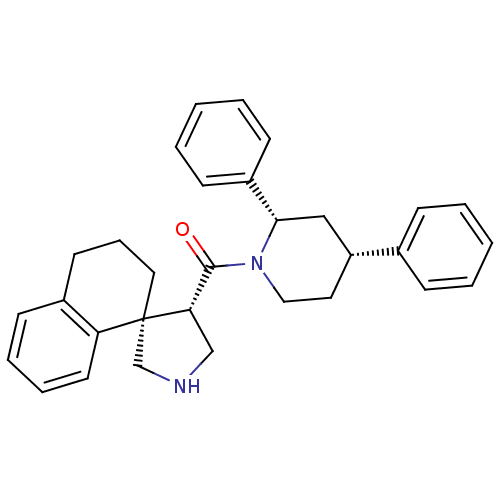 (CHEMBL1934292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16706 (5-(2-acetylphenyl)-1-N-[(2S,3R)-3-hydroxy-1-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16708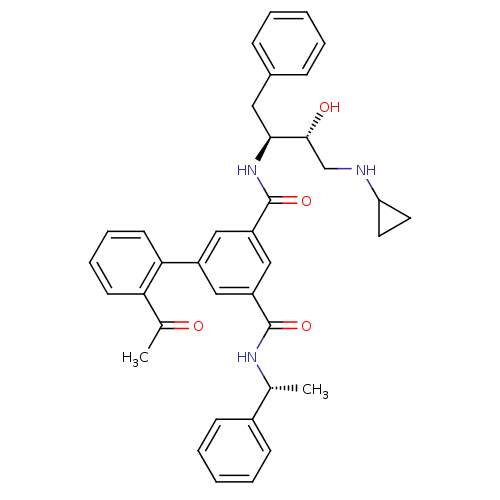 (1-N-[(2S,3R)-4-(cyclopropylamino)-3-hydroxy-1-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 317 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16714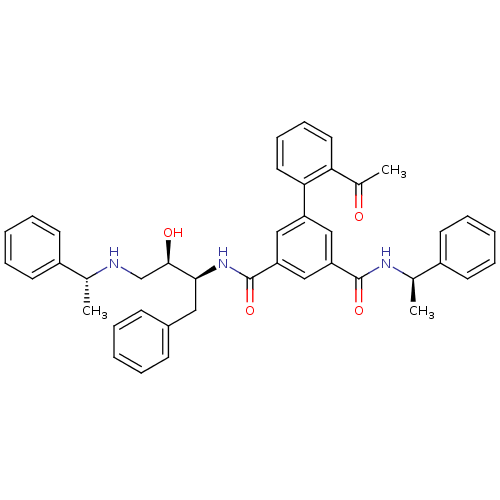 (5-(2-acetylphenyl)-1-N-[(2S,3R)-3-hydroxy-1-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 348 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16709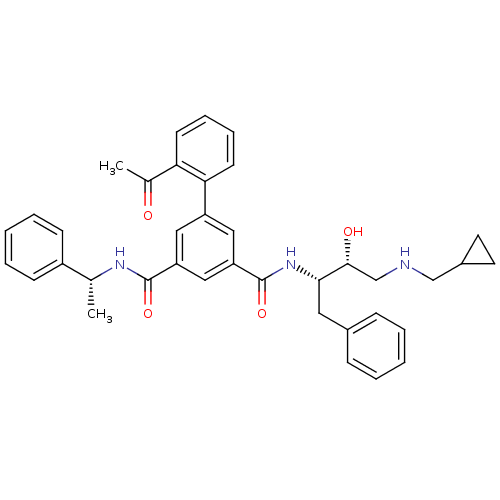 (1-N-[(2S,3R)-4-[(cyclopropylmethyl)amino]-3-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 418 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50258280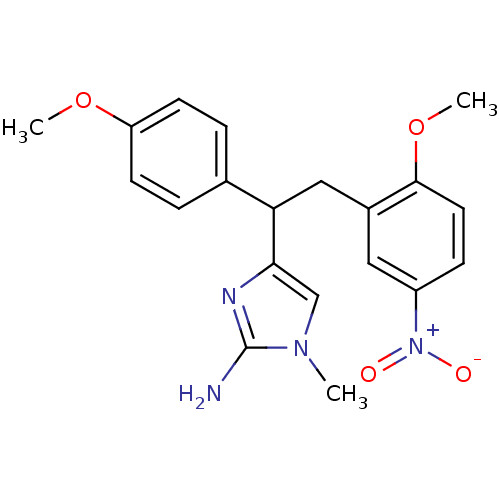 (4-(2-(2-methoxy-5-nitrophenyl)-1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) at pH 6.5 | Bioorg Med Chem Lett 19: 2977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.04.033 BindingDB Entry DOI: 10.7270/Q2XS5V96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360723 (CHEMBL1934295) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in mouse fibroblast cells assessed as inhibition of soluble APPbeta_NF cleavage | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360717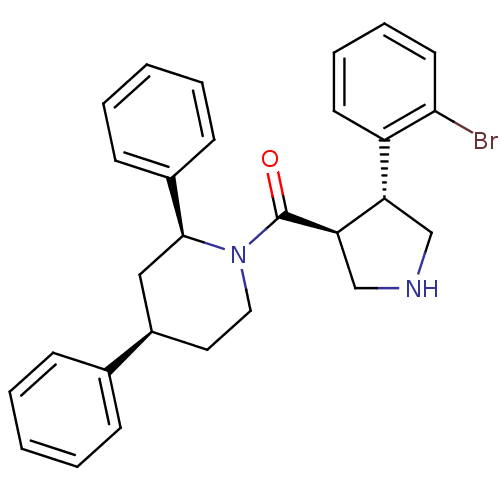 (CHEMBL1934289) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16689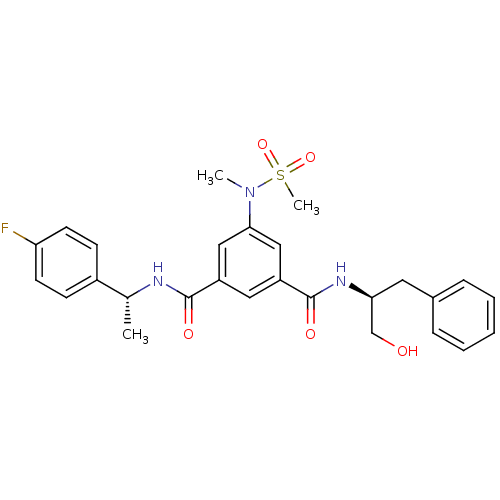 (1,3,5-trisubstituted aromatic scaffold, 2a | 1-N-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16707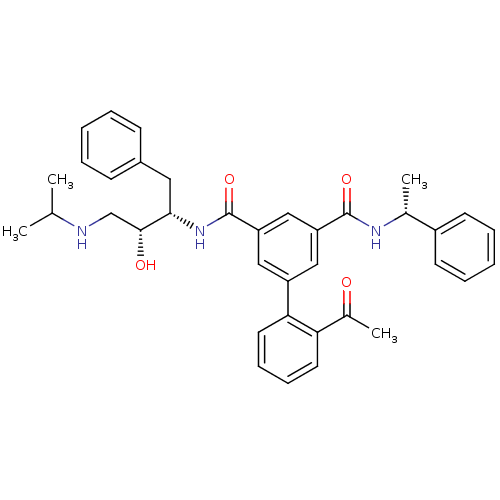 (5-(2-acetylphenyl)-1-N-[(2S,3R)-3-hydroxy-1-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 674 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50258849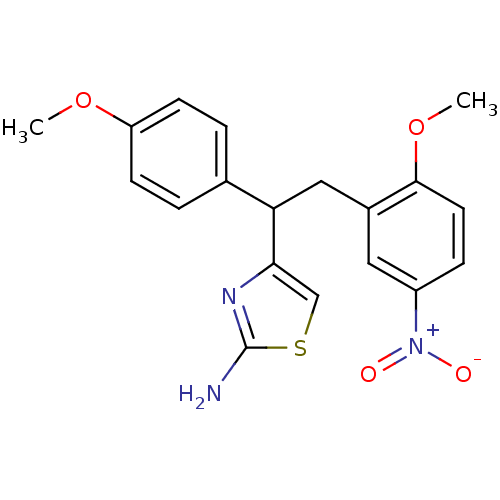 (4-(2-(2-methoxy-5-nitrophenyl)-1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) at pH 4.5 | Bioorg Med Chem Lett 19: 2977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.04.033 BindingDB Entry DOI: 10.7270/Q2XS5V96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16710 (1-N-[(2S,3R)-4-(cyclobutylamino)-3-hydroxy-1-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360722 (CHEMBL1934294) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in mouse fibroblast cells assessed as inhibition of soluble APPbeta_NF cleavage | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50258280 (4-(2-(2-methoxy-5-nitrophenyl)-1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) by sAPP_NF cell based assay | Bioorg Med Chem Lett 19: 2977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.04.033 BindingDB Entry DOI: 10.7270/Q2XS5V96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50258279 (4-(1-(3-fluoro-4-methoxyphenyl)-2-(2-methoxy-5-nit...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 6.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 at pH 6.4 | Bioorg Med Chem Lett 19: 2977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.04.033 BindingDB Entry DOI: 10.7270/Q2XS5V96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50258278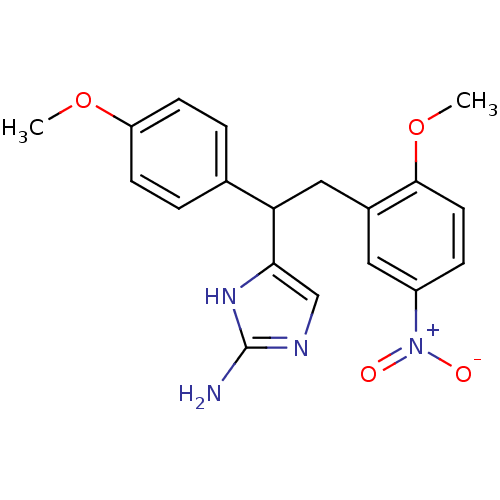 (4-(2-(2-methoxy-5-nitrophenyl)-1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) at pH 6.5 | Bioorg Med Chem Lett 19: 2977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.04.033 BindingDB Entry DOI: 10.7270/Q2XS5V96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360719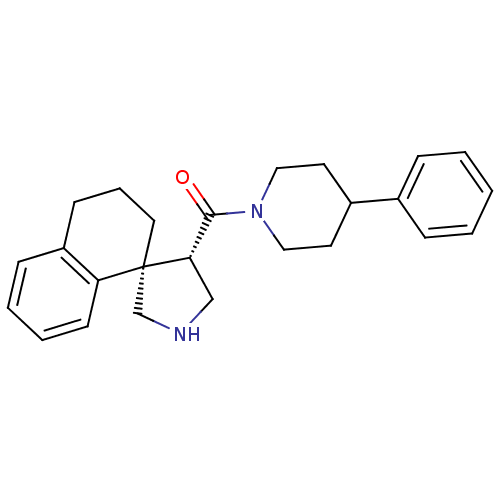 (CHEMBL1934291) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360714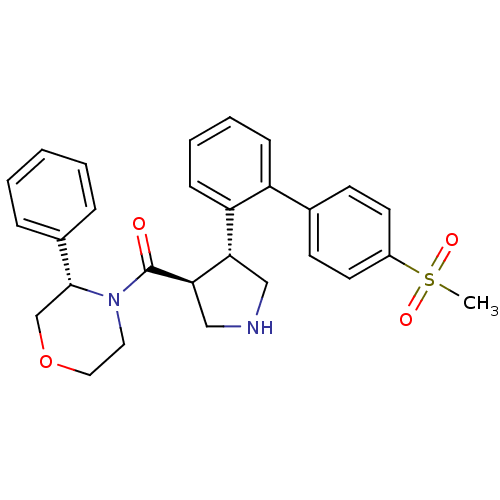 (CHEMBL1934286) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50258278 (4-(2-(2-methoxy-5-nitrophenyl)-1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) at pH 4.5 | Bioorg Med Chem Lett 19: 2977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.04.033 BindingDB Entry DOI: 10.7270/Q2XS5V96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16690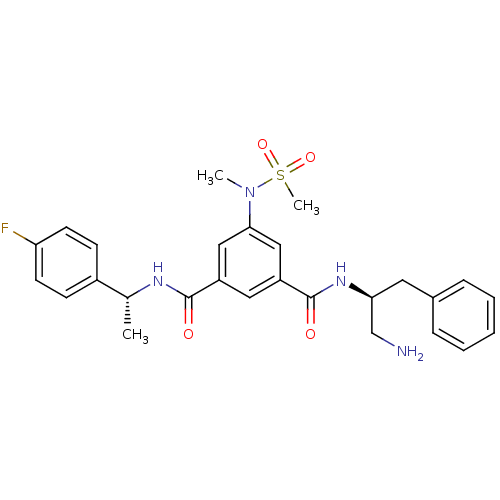 (1,3,5-trisubstituted aromatic scaffold, 2b | 1-N-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50360723 (CHEMBL1934295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Cat D | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16705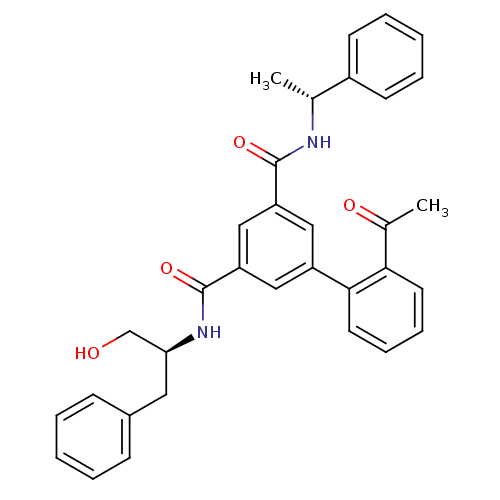 (5-(2-acetylphenyl)-1-N-[(2S)-1-hydroxy-3-phenylpro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16716 (1-N-[(2S,3R)-4-(azetidin-1-yl)-3-hydroxy-1-phenylb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16715 (1-N-[(2S,3R)-4-[benzyl(methyl)amino]-3-hydroxy-1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 823-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.051 BindingDB Entry DOI: 10.7270/Q2057D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16700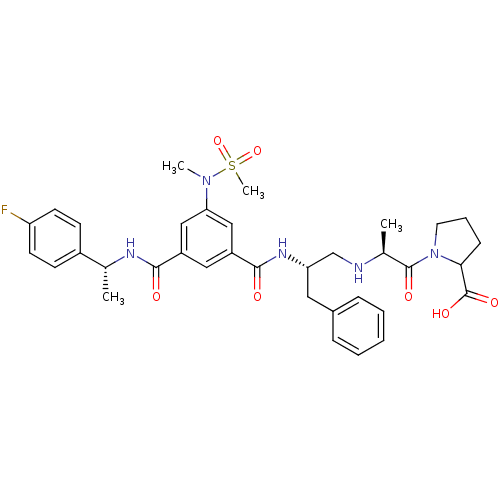 (1-[(2S)-2-{[(2S)-2-[(3-{[(1R)-1-(4-fluorophenyl)et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360716 (CHEMBL1934288) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360718 (CHEMBL1934290) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50258847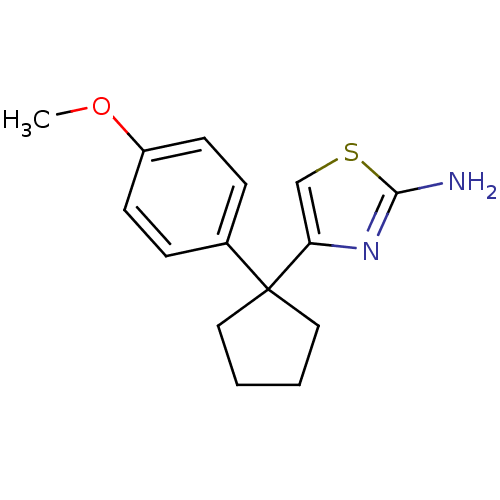 (4-(1-(4-methoxyphenyl)cyclopentyl)thiazol-2-amine ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) at pH 4.5 | Bioorg Med Chem Lett 19: 2977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.04.033 BindingDB Entry DOI: 10.7270/Q2XS5V96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50258848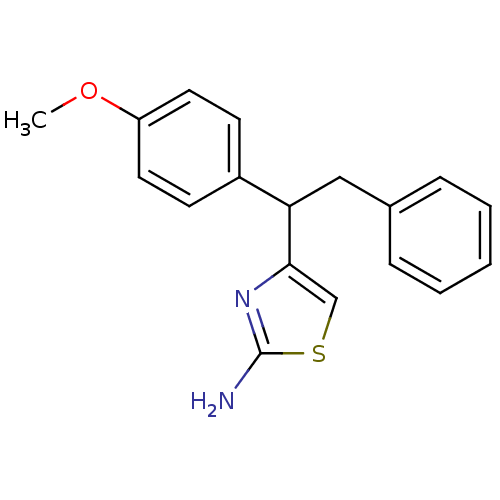 (4-(1-(4-methoxyphenyl)-2-phenylethyl)thiazol-2-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) at pH 4.5 | Bioorg Med Chem Lett 19: 2977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.04.033 BindingDB Entry DOI: 10.7270/Q2XS5V96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50258846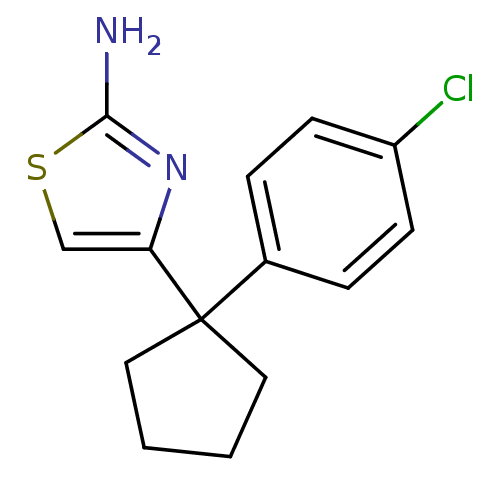 (4-(1-(4-chlorophenyl)cyclopentyl)thiazol-2-amine |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) at pH 4.5 | Bioorg Med Chem Lett 19: 2977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.04.033 BindingDB Entry DOI: 10.7270/Q2XS5V96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50360713 (CHEMBL1934285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in cell-free system | Bioorg Med Chem Lett 22: 240-4 (2011) Article DOI: 10.1016/j.bmcl.2011.11.024 BindingDB Entry DOI: 10.7270/Q2QR4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |