

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50399088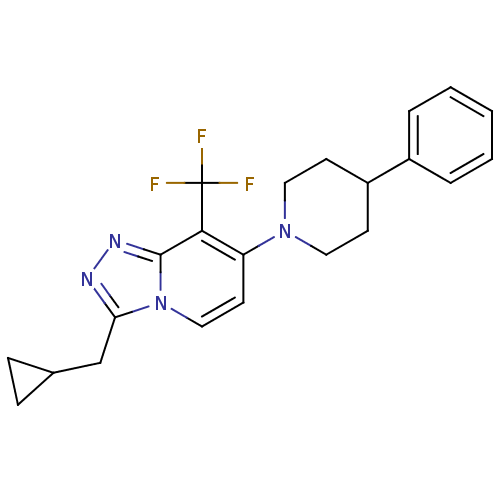 (CHEMBL2179319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]8-Trifluoromethyl-3-cyclopropylmethyl-7-[(4-phenyl-1-piperidinyl)methyl]-1,2,4-triazolo[4,3-a]pyridine from mGlu2 receptor (unkno... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194617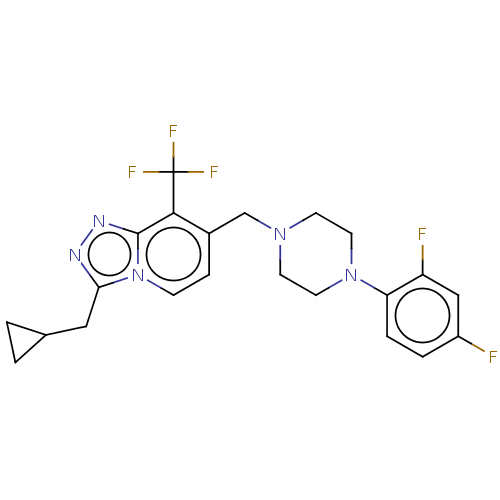 (CHEMBL3926416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]8-Trifluoromethyl-3-cyclopropylmethyl-7-[(4-phenyl-1-piperidinyl)methyl]-1,2,4-triazolo[4,3-a]pyridine from mGlu2 receptor (unkno... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50051408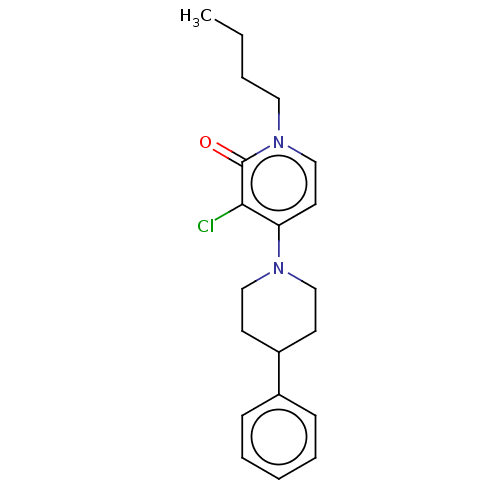 (CHEMBL3337527 | US11071729, Compound 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]8-Trifluoromethyl-3-cyclopropylmethyl-7-[(4-phenyl-1-piperidinyl)methyl]-1,2,4-triazolo[4,3-a]pyridine from mGlu2 receptor (unkno... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292582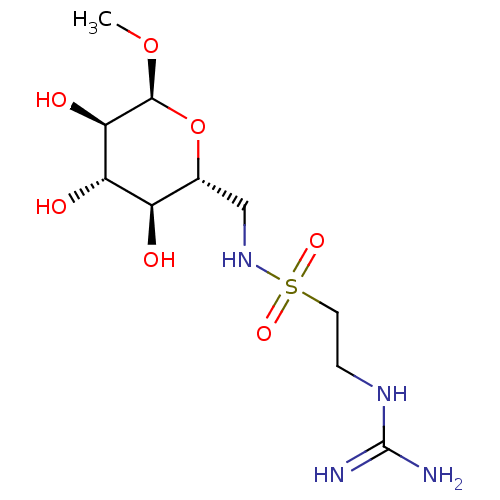 (CHEMBL4162427) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292584 (CHEMBL4172694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292583 (CHEMBL4164794) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292582 (CHEMBL4162427) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292585 (CHEMBL4161382) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292585 (CHEMBL4161382) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of VLA-4 expressed in Jurkat cell line, in a cell-based adhesion assay. | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292584 (CHEMBL4172694) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292590 (CHEMBL4162165) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292586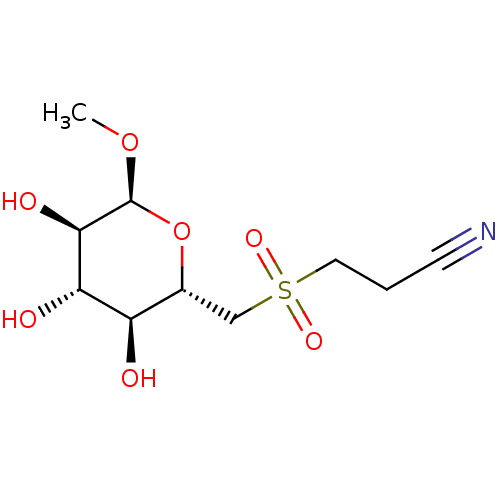 (CHEMBL4169301) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292580 (CHEMBL4172838) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292587 (CHEMBL4167547) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292586 (CHEMBL4169301) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292590 (CHEMBL4162165) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292589 (CHEMBL4159624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292589 (CHEMBL4159624) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292587 (CHEMBL4167547) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292583 (CHEMBL4164794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292580 (CHEMBL4172838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50399088 (CHEMBL2179319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Binding affinity to human mGlu2R expressed in CHO cells by radioligand binding assay | J Med Chem 55: 8770-89 (2012) Article DOI: 10.1021/jm3010724 BindingDB Entry DOI: 10.7270/Q26111FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50051408 (CHEMBL3337527 | US11071729, Compound 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Displacement of [3H]19 from human mGlu2 receptor expressed in CHO cells | J Med Chem 57: 6495-512 (2014) Article DOI: 10.1021/jm500496m BindingDB Entry DOI: 10.7270/Q2DJ5H8J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292581 (CHEMBL4165870) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by Lineweaver-Burk plot analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50511341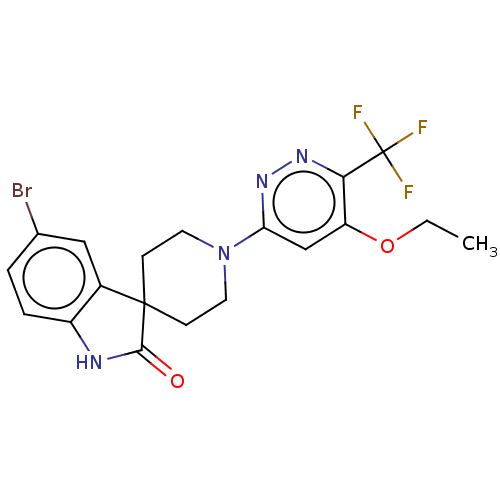 (CHEMBL4483761) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Displacement of [3H]RX 821002 from human recombinant adrenergic alpha-2B receptor expressed in CHO cells | ACS Med Chem Lett 11: 303-308 (2020) Article DOI: 10.1021/acsmedchemlett.9b00350 BindingDB Entry DOI: 10.7270/Q2GB27DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50396489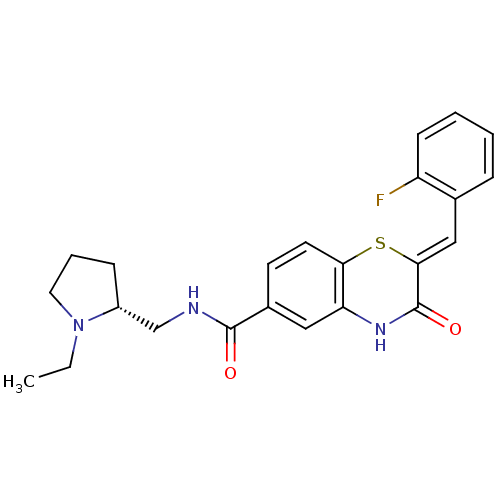 (CHEMBL2170943) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by Lineweaver-Burk plot analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50399088 (CHEMBL2179319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 55: 8770-89 (2012) Article DOI: 10.1021/jm3010724 BindingDB Entry DOI: 10.7270/Q26111FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50399088 (CHEMBL2179319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 55: 8770-89 (2012) Article DOI: 10.1021/jm3010724 BindingDB Entry DOI: 10.7270/Q26111FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50194617 (CHEMBL3926416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50194617 (CHEMBL3926416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50194617 (CHEMBL3926416) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50194617 (CHEMBL3926416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50194617 (CHEMBL3926416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50194617 (CHEMBL3926416) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50399088 (CHEMBL2179319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 55: 8770-89 (2012) Article DOI: 10.1021/jm3010724 BindingDB Entry DOI: 10.7270/Q26111FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50399088 (CHEMBL2179319) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 55: 8770-89 (2012) Article DOI: 10.1021/jm3010724 BindingDB Entry DOI: 10.7270/Q26111FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50399088 (CHEMBL2179319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Inhibition of CYP1A2 | J Med Chem 55: 8770-89 (2012) Article DOI: 10.1021/jm3010724 BindingDB Entry DOI: 10.7270/Q26111FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50396489 (CHEMBL2170943) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by Linew... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292581 (CHEMBL4165870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by Linew... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50415239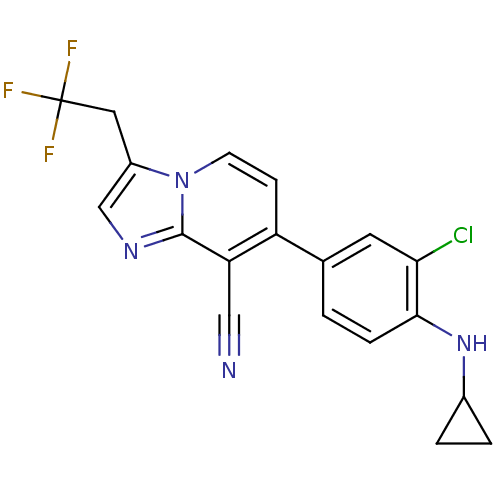 (CHEMBL593168) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a |
Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Allosteric modulation of human mGluR2 expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 20: 175-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.008 BindingDB Entry DOI: 10.7270/Q2W95BD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50393310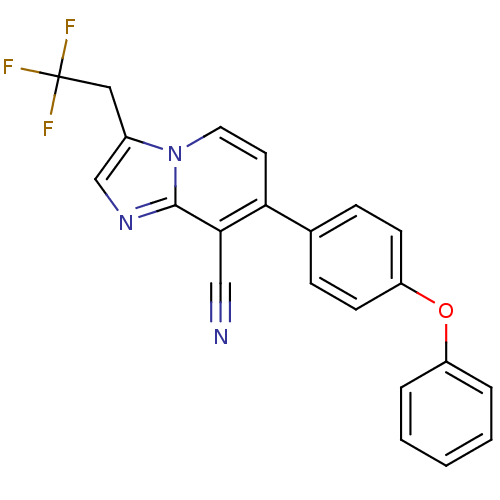 (CHEMBL605921) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 186 | n/a | n/a | n/a | n/a |
Janssen-Cilag S.A. Curated by ChEMBL | Assay Description Allosteric modulation of human mGluR2 expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 20: 175-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.008 BindingDB Entry DOI: 10.7270/Q2W95BD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194613 (CHEMBL3947764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Positive allosteric modulation of human mGlu2 receptor expressed in CHO cell membranes assessed as potentiation of glutamate-induced effect incubated... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194614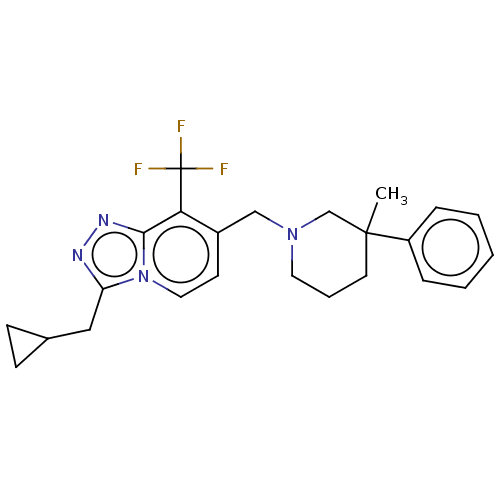 (CHEMBL3901998) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Positive allosteric modulation of human mGlu2 receptor expressed in CHO cell membranes assessed as potentiation of glutamate-induced effect incubated... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194615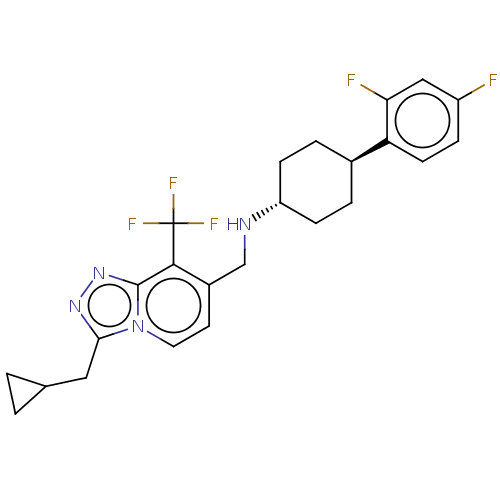 (CHEMBL3940264) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Positive allosteric modulation of human mGlu2 receptor expressed in CHO cell membranes assessed as potentiation of glutamate-induced effect incubated... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194616 (CHEMBL3956434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Positive allosteric modulation of human mGlu2 receptor expressed in CHO cell membranes assessed as potentiation of glutamate-induced effect incubated... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194617 (CHEMBL3926416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Positive allosteric modulation of human mGlu2 receptor expressed in CHO cell membranes assessed as potentiation of glutamate-induced effect incubated... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194618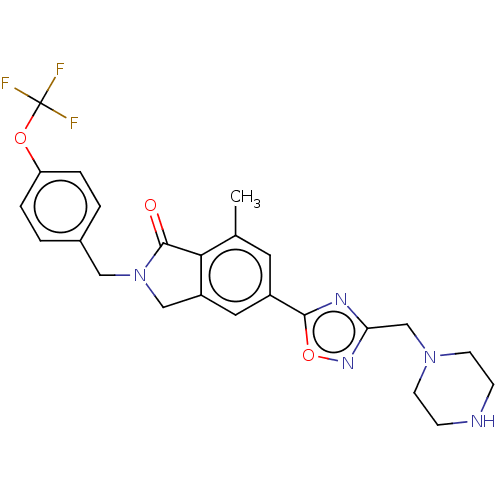 (CHEMBL3937907 | US20240050427, Compound 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 195 | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Positive allosteric modulation of human mGlu2 receptor expressed in CHO cell membranes assessed as potentiation of glutamate-induced effect incubated... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194662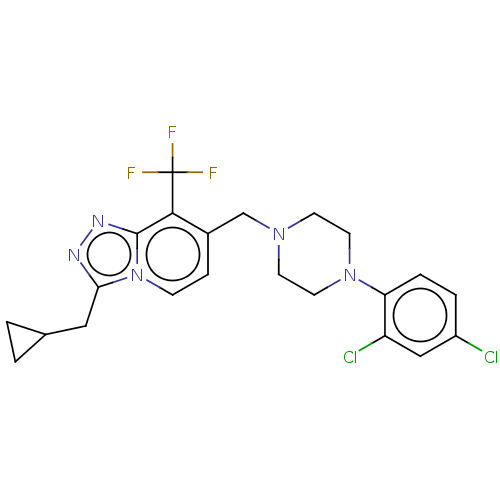 (CHEMBL3957311) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Positive allosteric modulation of human mGlu2 receptor expressed in CHO cell membranes assessed as potentiation of glutamate-induced effect incubated... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50399088 (CHEMBL2179319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Positive allosteric modulation of human mGlu2 receptor expressed in CHO cell membranes assessed as potentiation of glutamate-induced effect incubated... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194664 (CHEMBL3919978) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 132 | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Positive allosteric modulation of human mGlu2 receptor expressed in CHO cell membranes assessed as potentiation of glutamate-induced effect incubated... | J Med Chem 59: 8495-507 (2016) Article DOI: 10.1021/acs.jmedchem.6b00913 BindingDB Entry DOI: 10.7270/Q27M09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 193 total ) | Next | Last >> |