

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50231951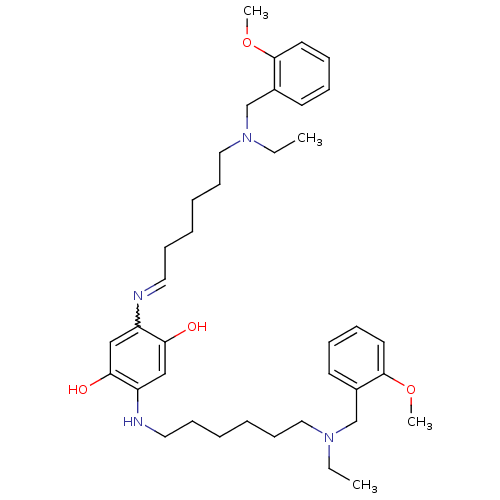 (2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50500613 (CHEMBL3752642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Inhibition of Cyclophilin A peptidyl-prolyl cis-trans isomerase activity (unknown origin) using Succ-Ala-Leu-Pro-Phe-p-nitroaniline as substrate by I... | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Inhibition of Cyclophilin A peptidyl-prolyl cis-trans isomerase activity (unknown origin) using Succ-Ala-Leu-Pro-Phe-p-nitroaniline as substrate by I... | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50500614 (CHEMBL3754544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Inhibition of Cyclophilin A peptidyl-prolyl cis-trans isomerase activity (unknown origin) using Succ-Ala-Leu-Pro-Phe-p-nitroaniline as substrate by I... | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361663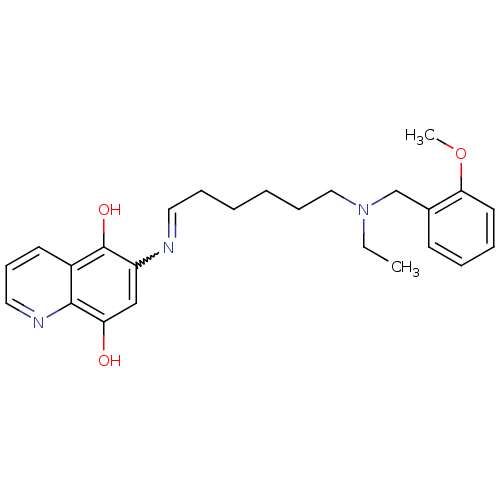 (CHEMBL1940613) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361664 (CHEMBL1940614) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361665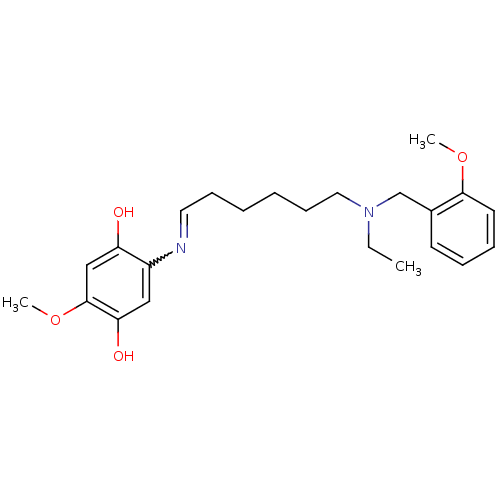 (CHEMBL1940615) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50231951 (2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50361664 (CHEMBL1940614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50361662 (CHEMBL1940612) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361666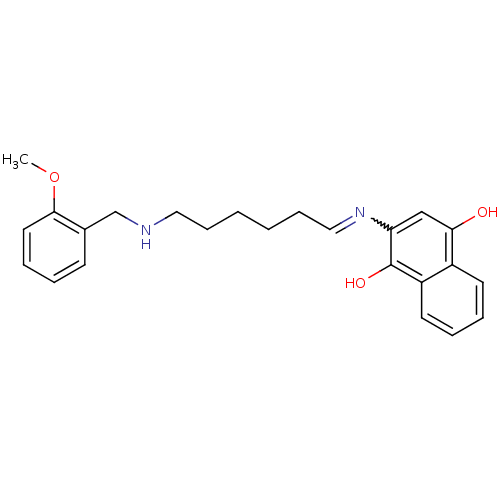 (CHEMBL1940616) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50361663 (CHEMBL1940613) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361662 (CHEMBL1940612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in baculovirus system using panvera peptide as substrate after 60 mins by spectrofluorometric analysis | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361662 (CHEMBL1940612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in baculovirus system using M-2420 as substrate preincubated for 1 hr before substrate addition measured af... | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50361668 (CHEMBL1940618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50361666 (CHEMBL1940616) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50361669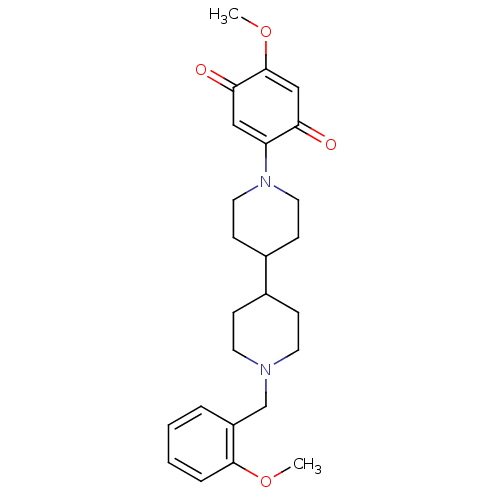 (CHEMBL1940619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50231951 (2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in baculovirus system using M-2420 as substrate preincubated for 1 hr before substrate addition measured af... | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361668 (CHEMBL1940618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50361665 (CHEMBL1940615) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361669 (CHEMBL1940619) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361662 (CHEMBL1940612) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361662 (CHEMBL1940612) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE-induced amyloid beta (1-40) aggregation assessed as fibril formation after 24 hrs by thioflavin T-based fluorome... | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361667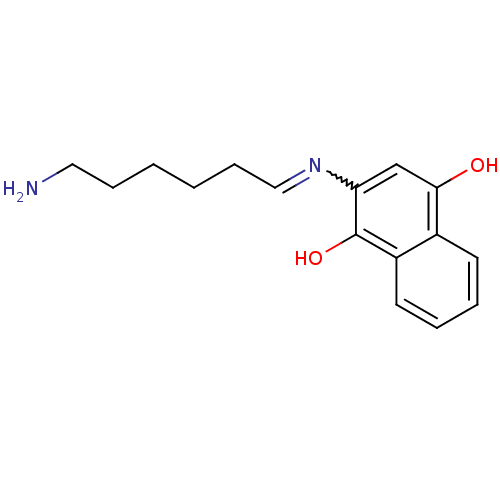 (CHEMBL1940617) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50361667 (CHEMBL1940617) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide as substrate after 20 mins preincubation by Ellman method | J Med Chem 54: 8299-304 (2011) Article DOI: 10.1021/jm200691d BindingDB Entry DOI: 10.7270/Q2V40VNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50500613 (CHEMBL3752642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Binding affinity to Cyclophilin A (unknown origin) at 0.625 nM to 10 uM by surface plasmon resonance analysis | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50500613 (CHEMBL3752642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Binding affinity to Cyclophilin B (unknown origin) by surface plasmon resonance analysis | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50500613 (CHEMBL3752642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Binding affinity to Cyclophilin A (unknown origin) at 0.625 nM to 10 uM by surface plasmon resonance analysis | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Binding affinity to Cyclophilin B (unknown origin) by surface plasmon resonance analysis | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50500613 (CHEMBL3752642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Binding affinity to Cyclophilin A (unknown origin) by surface plasmon resonance analysis | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50500613 (CHEMBL3752642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Binding affinity to Cyclophilin B (unknown origin) at 10 to 30 uM by surface plasmon resonance analysis | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50500613 (CHEMBL3752642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Binding affinity to Cyclophilin B (unknown origin) at 10 to 30 uM by surface plasmon resonance analysis | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Binding affinity to Cyclophilin A (unknown origin) by surface plasmon resonance analysis | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||