

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548115 (CHEMBL4778773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548110 (CHEMBL4747532) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50162075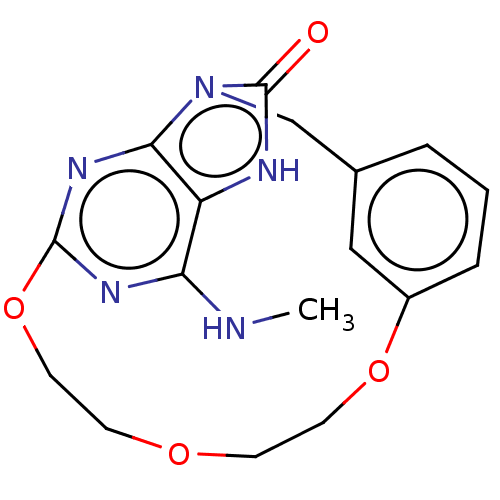 (CHEMBL3794167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50162074 (CHEMBL3792684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548117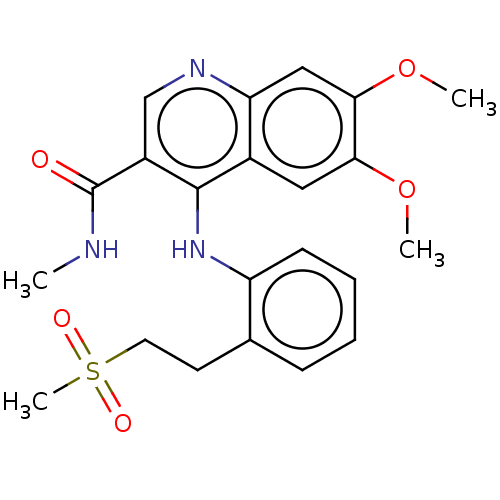 (CHEMBL4776026) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548124 (CHEMBL4784602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548109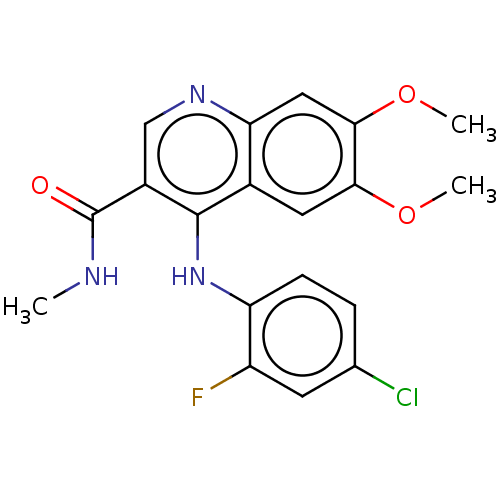 (CHEMBL4783492) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50580186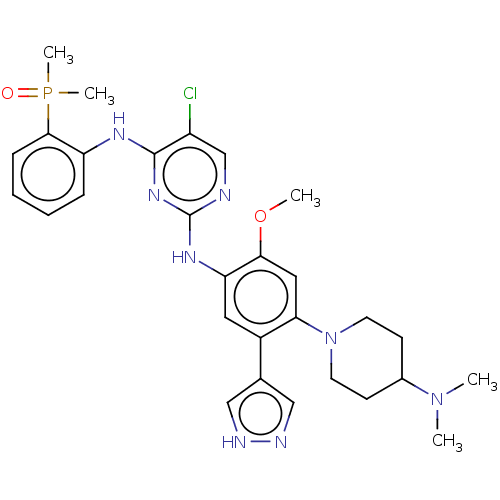 (CHEMBL5094480) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Wild type EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50580187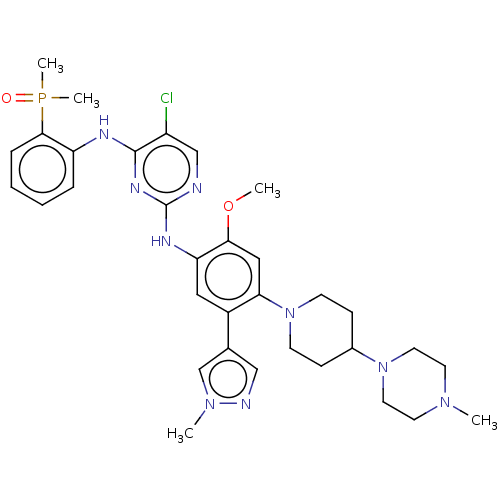 (CHEMBL5087815) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Wild type EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50580185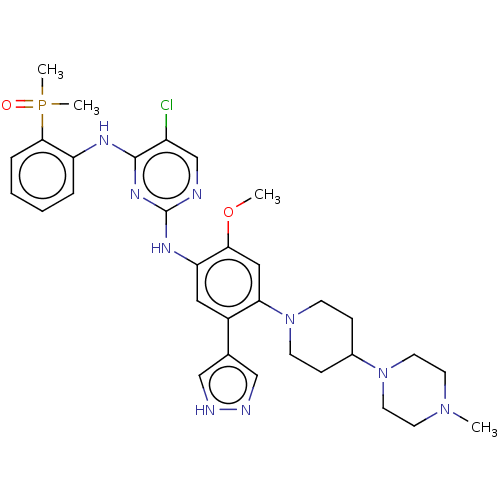 (CHEMBL5077378) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Wild type EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548121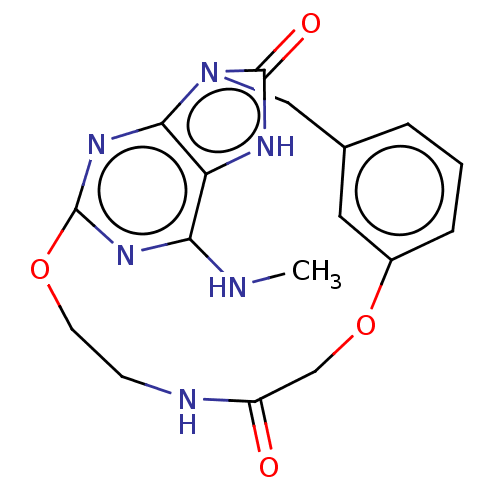 (CHEMBL4754128) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50580188 (CHEMBL5086066) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Wild type EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548119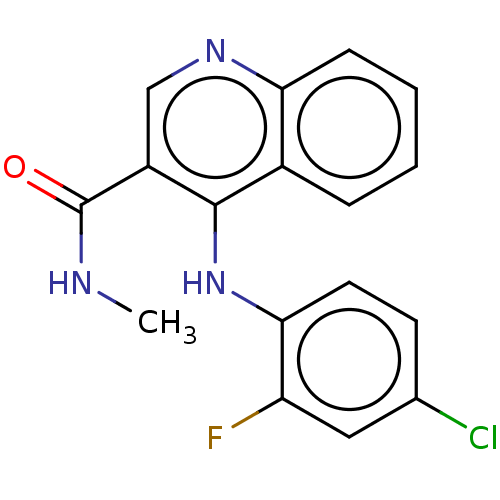 (CHEMBL4749068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50580192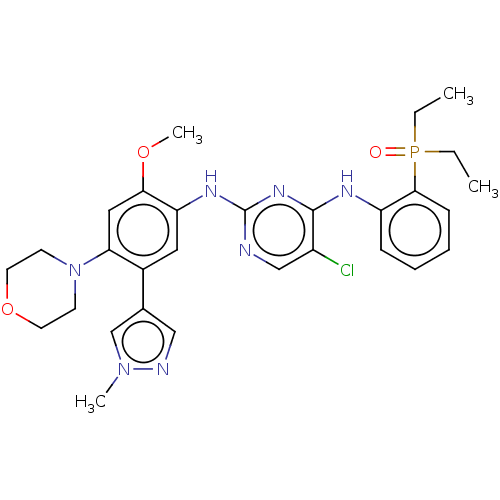 (CHEMBL5078413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Wild type EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152124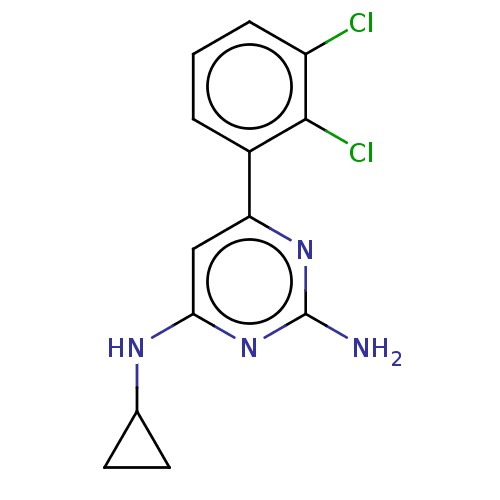 (CHEMBL3782004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50580189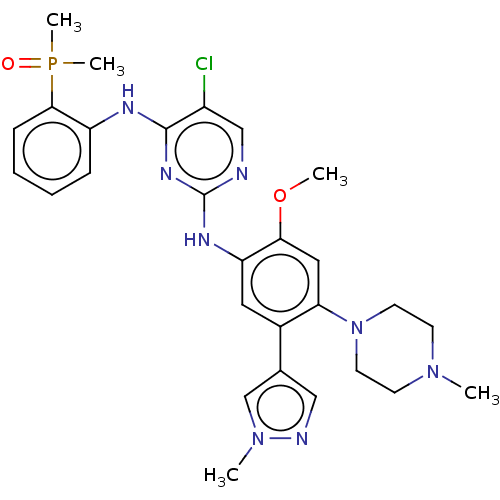 (CHEMBL5087610) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Wild type EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50580190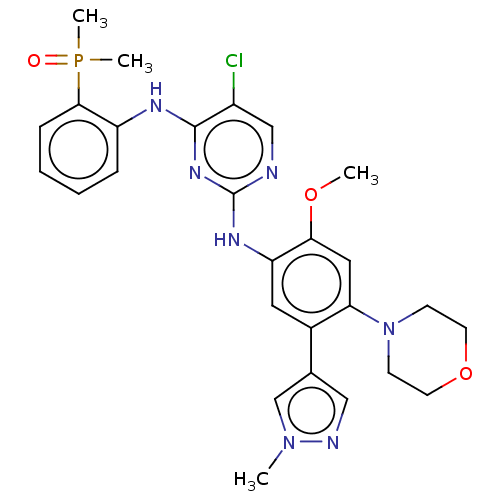 (CHEMBL5087264) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Wild type EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50580191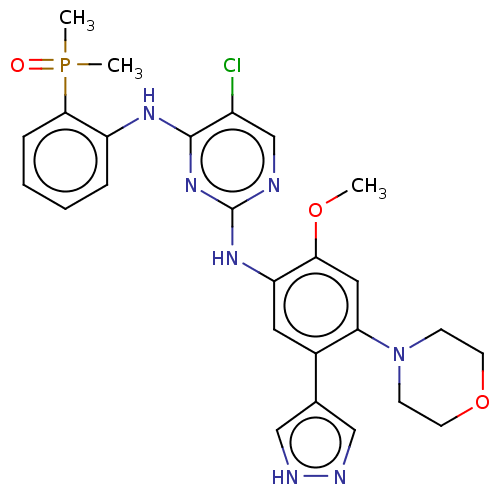 (CHEMBL5081299) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Wild type EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548107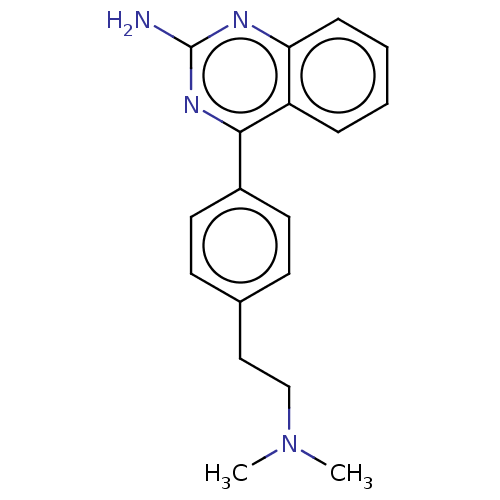 (CHEMBL4799076) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548118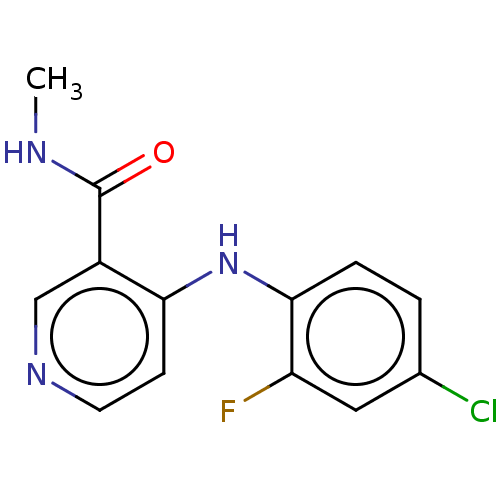 (CHEMBL4758926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255420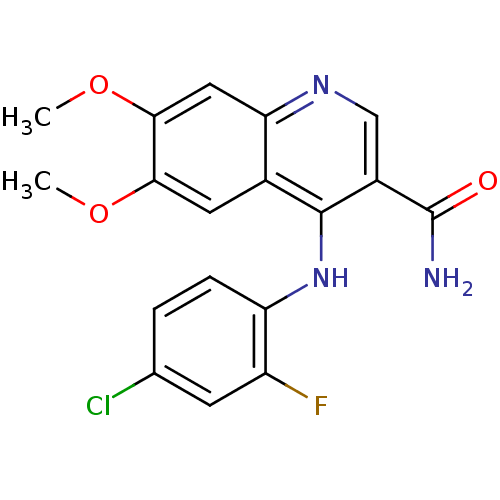 (4-(4-chloro-2-fluorophenylamino)-6,7-dimethoxyquin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50185140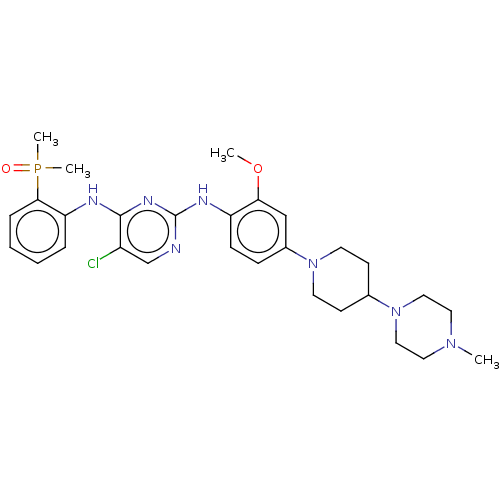 (AP-26113 | Brigatinib | US11248003, Example Brigat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Wild type EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548112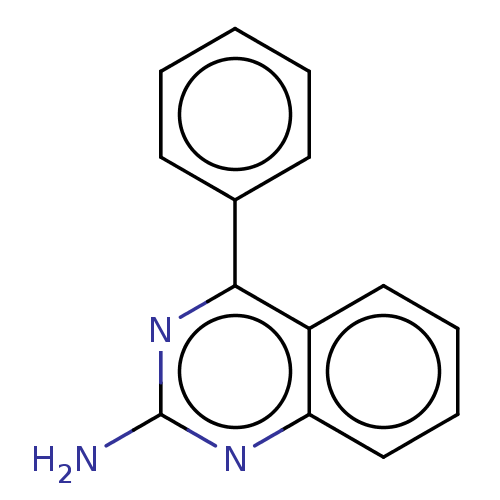 (CHEMBL4791612) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50580190 (CHEMBL5087264) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EGFR L858R/T790M mutant (unknown origin) expressed in human NCI-H1975 cells assessed as protein phosphorylation measured after 2 hrs by... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239385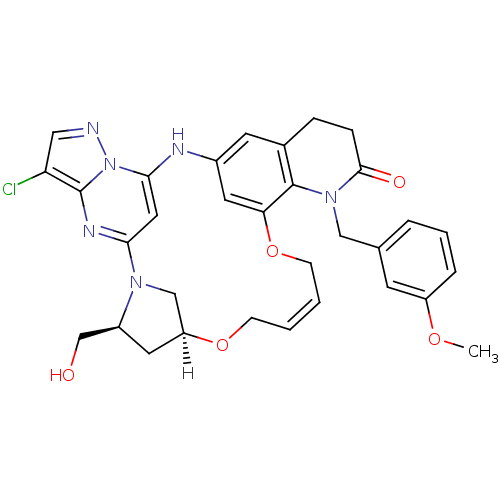 (CHEMBL4092565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor in guinea pig cerebral cortical membranes by displacement of [3H]- WB-4101 | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239368 (CHEMBL4073463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50352564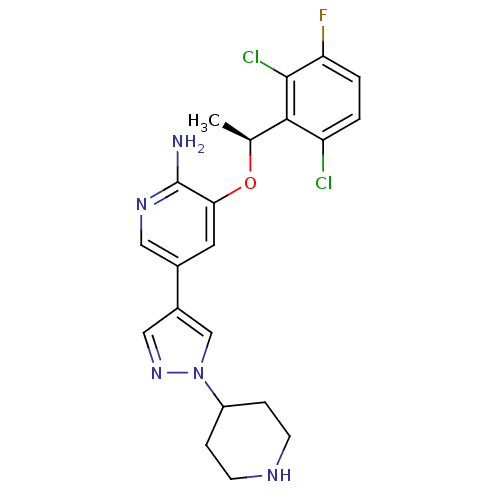 (CHEMBL1825141) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548122 (CHEMBL4743321) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239368 (CHEMBL4073463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548123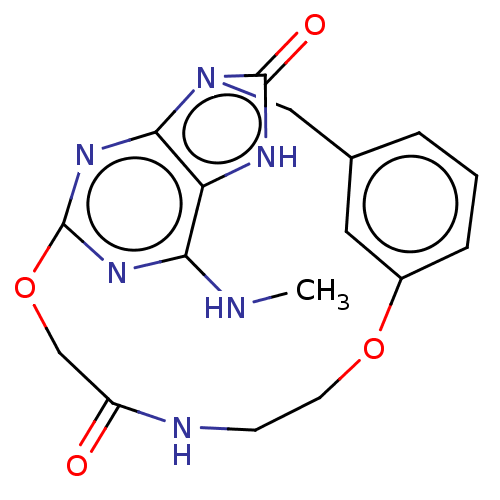 (CHEMBL4791011) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239362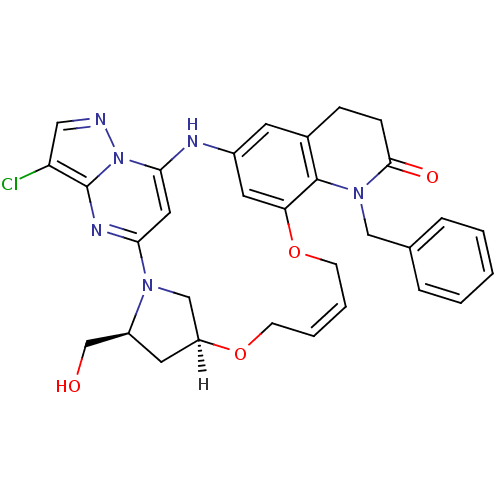 (CHEMBL4064865) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239362 (CHEMBL4064865) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50185140 (AP-26113 | Brigatinib | US11248003, Example Brigat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EGFR L858R/T790M mutant (unknown origin) expressed in human NCI-H1975 cells assessed as protein phosphorylation measured after 2 hrs by... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01055 BindingDB Entry DOI: 10.7270/Q2959NF7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239380 (CHEMBL4062105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239380 (CHEMBL4062105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239383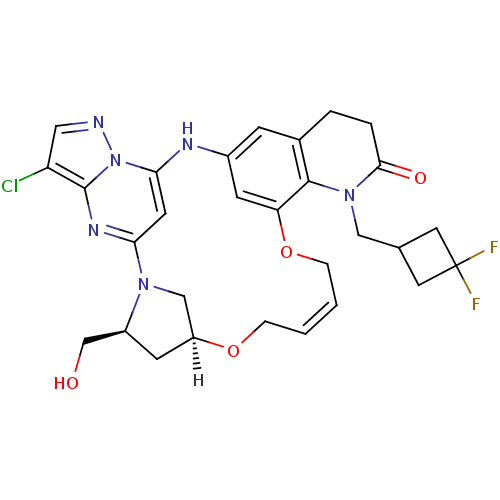 (CHEMBL4096773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239383 (CHEMBL4096773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548111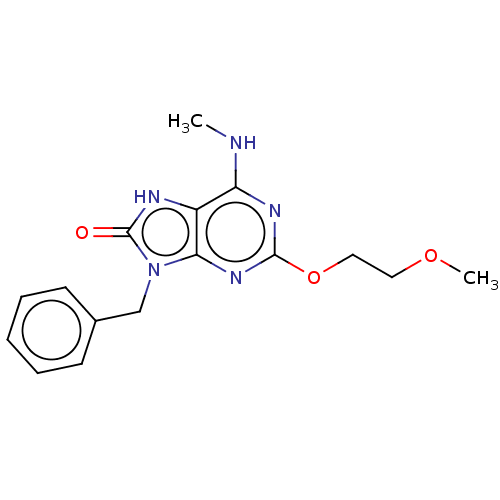 (CHEMBL4763327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 536 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50240849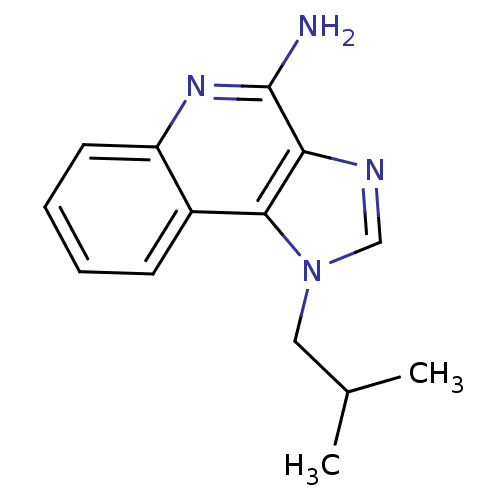 (1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239379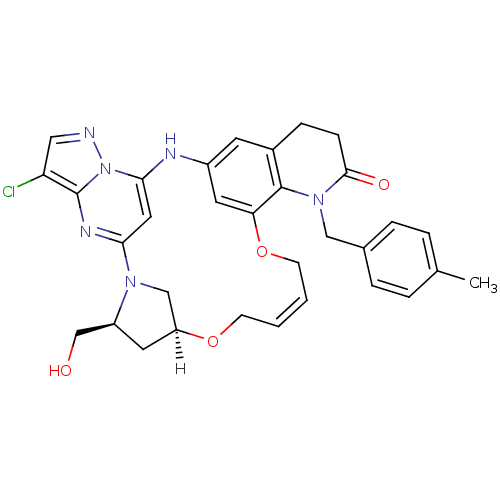 (CHEMBL4083842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239379 (CHEMBL4083842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548120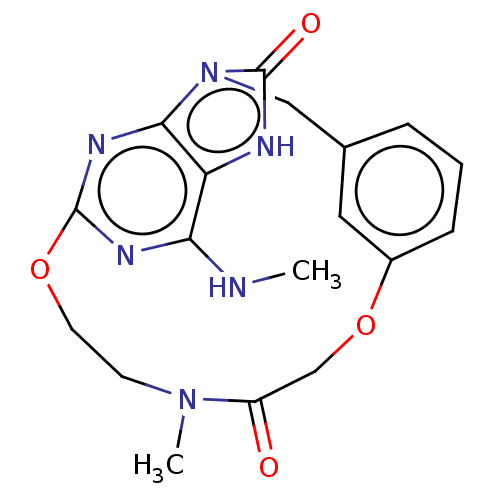 (CHEMBL4747421) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 888 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239367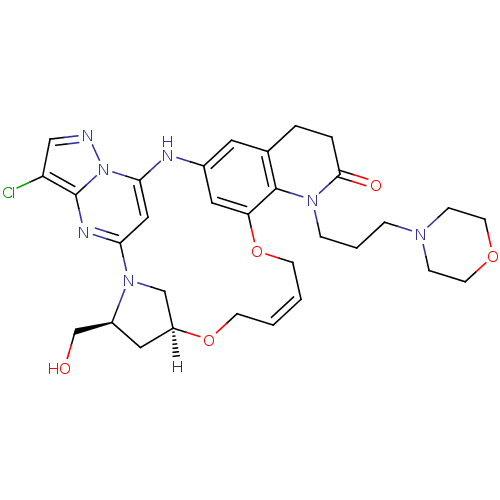 (CHEMBL4084806) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548108 (CHEMBL4749745) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239367 (CHEMBL4084806) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239363 (CHEMBL4094351) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50241029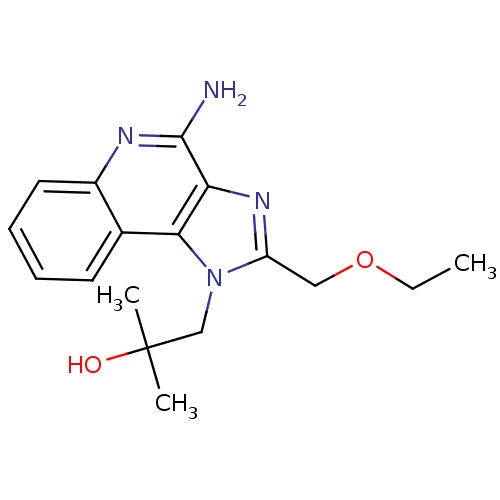 (1-[4-amino-2-(ethoxymethyl)-1H-imidazo[4,5-c]quino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50189516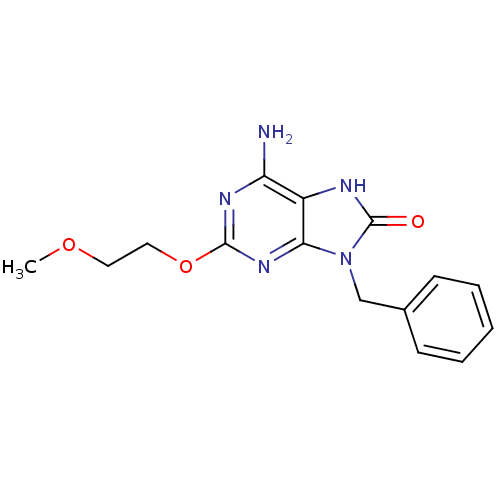 (6-amino-9-benzyl-2-(2-methoxyethoxy)-7H-purin-8(9H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 104 total ) | Next | Last >> |