 Found 29 hits with Last Name = 'akoachere' and Initial = 'mb'
Found 29 hits with Last Name = 'akoachere' and Initial = 'mb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182122

(CHEMBL204688 | bis(2,4-dinitrophenyl)sulfane)Show SMILES [O-][N+](=O)c1ccc(Sc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C12H6N4O8S/c17-13(18)7-1-3-11(9(5-7)15(21)22)25-12-4-2-8(14(19)20)6-10(12)16(23)24/h1-6H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to Plasmodium falciparum TrxR in presence of thioredoxin |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182122

(CHEMBL204688 | bis(2,4-dinitrophenyl)sulfane)Show SMILES [O-][N+](=O)c1ccc(Sc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C12H6N4O8S/c17-13(18)7-1-3-11(9(5-7)15(21)22)25-12-4-2-8(14(19)20)6-10(12)16(23)24/h1-6H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to Plasmodium falciparum TrxR in presence of NADPH |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182128

(4-nitrobenzo[c][1,2,5]thiadiazole | CHEMBL383084)Show InChI InChI=1S/C6H3N3O2S/c10-9(11)5-3-1-2-4-6(5)8-12-7-4/h1-3H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to Plasmodium falciparum TrxR in presence of thioredoxin |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182128

(4-nitrobenzo[c][1,2,5]thiadiazole | CHEMBL383084)Show InChI InChI=1S/C6H3N3O2S/c10-9(11)5-3-1-2-4-6(5)8-12-7-4/h1-3H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to Plasmodium falciparum TrxR in presence of NADPH |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182122

(CHEMBL204688 | bis(2,4-dinitrophenyl)sulfane)Show SMILES [O-][N+](=O)c1ccc(Sc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C12H6N4O8S/c17-13(18)7-1-3-11(9(5-7)15(21)22)25-12-4-2-8(14(19)20)6-10(12)16(23)24/h1-6H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182128

(4-nitrobenzo[c][1,2,5]thiadiazole | CHEMBL383084)Show InChI InChI=1S/C6H3N3O2S/c10-9(11)5-3-1-2-4-6(5)8-12-7-4/h1-3H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182124
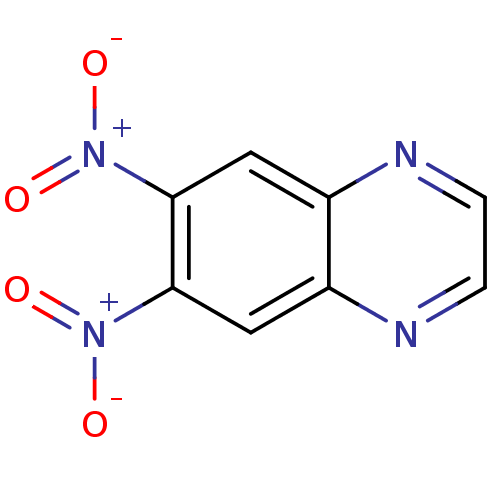
(6,7-dinitroquinoxaline | CHEMBL380953)Show InChI InChI=1S/C8H4N4O4/c13-11(14)7-3-5-6(10-2-1-9-5)4-8(7)12(15)16/h1-4H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50182130
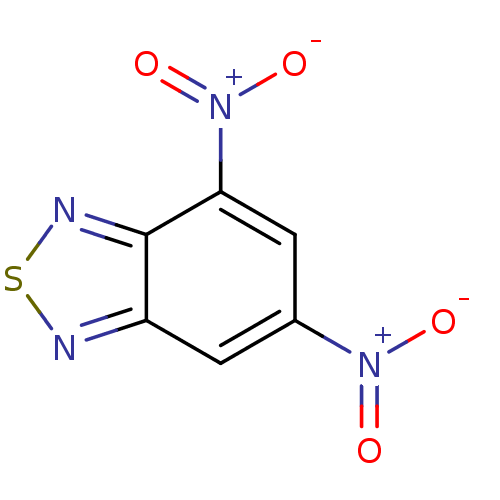
(4,6-dinitrobenzo[c][1,2,5]thiadiazole | CHEMBL2061...)Show InChI InChI=1S/C6H2N4O4S/c11-9(12)3-1-4-6(8-15-7-4)5(2-3)10(13)14/h1-2H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50182122

(CHEMBL204688 | bis(2,4-dinitrophenyl)sulfane)Show SMILES [O-][N+](=O)c1ccc(Sc2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C12H6N4O8S/c17-13(18)7-1-3-11(9(5-7)15(21)22)25-12-4-2-8(14(19)20)6-10(12)16(23)24/h1-6H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50068343
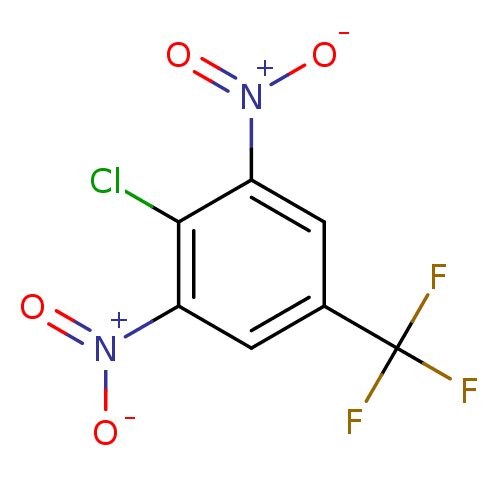
(2-Chloro-1,3-dinitro-5-trifluoromethyl-benzene | 2...)Show SMILES [O-][N+](=O)c1cc(cc(c1Cl)[N+]([O-])=O)C(F)(F)F Show InChI InChI=1S/C7H2ClF3N2O4/c8-6-4(12(14)15)1-3(7(9,10)11)2-5(6)13(16)17/h1-2H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182121

(4,5-dinitrobenzo[d][1,3]dioxole | CHEMBL206829)Show InChI InChI=1S/C7H4N2O6/c10-8(11)4-1-2-5-7(15-3-14-5)6(4)9(12)13/h1-2H,3H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182130

(4,6-dinitrobenzo[c][1,2,5]thiadiazole | CHEMBL2061...)Show InChI InChI=1S/C6H2N4O4S/c11-9(12)3-1-4-6(8-15-7-4)5(2-3)10(13)14/h1-2H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182129
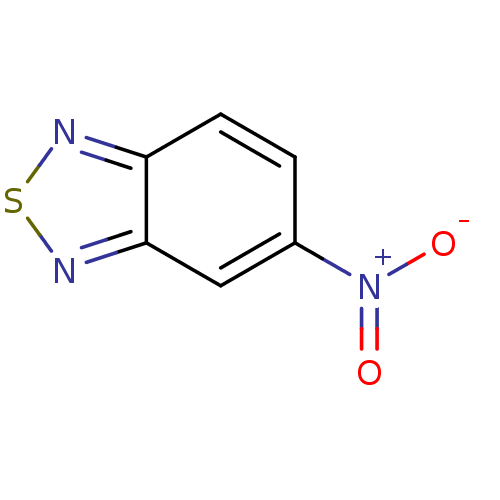
(5-nitrobenzo[c][1,2,5]thiadiazole | CHEMBL206713)Show InChI InChI=1S/C6H3N3O2S/c10-9(11)4-1-2-5-6(3-4)8-12-7-5/h1-3H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Glutathione reductase, mitochondrial
(Homo sapiens (Human)) | BDBM50241461

(3,7-bis(dimethylamino)phenothiazin-5-ium chloride ...)Show SMILES CN(C)c1ccc2nc3ccc(cc3sc2c1)=[N+](C)C |(17.73,-40.24,;19.06,-41.01,;19.06,-42.55,;20.39,-40.24,;20.4,-38.7,;21.73,-37.93,;23.06,-38.69,;24.39,-37.91,;25.73,-38.69,;27.06,-37.93,;28.39,-38.69,;28.39,-40.23,;27.06,-41,;25.73,-40.23,;24.39,-41.01,;23.06,-40.24,;21.73,-41.01,;29.73,-41,;29.73,-42.54,;31.06,-40.23,)| Show InChI InChI=1S/C16H18N3S/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13/h5-10H,1-4H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | 6.9 | n/a |
Biochemie-Zentrum der Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant glutathione reductase at pH 6.9 |
Antimicrob Agents Chemother 52: 183-91 (2007)
Article DOI: 10.1128/AAC.00773-07
BindingDB Entry DOI: 10.7270/Q21V5F0T |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182125
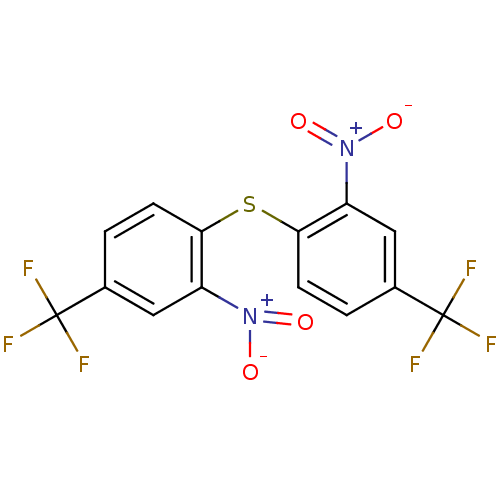
(CHEMBL204687 | bis(2-nitro-4-(trifluoromethyl)phen...)Show SMILES [O-][N+](=O)c1cc(ccc1Sc1ccc(cc1[N+]([O-])=O)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C14H6F6N2O4S/c15-13(16,17)7-1-3-11(9(5-7)21(23)24)27-12-4-2-8(14(18,19)20)6-10(12)22(25)26/h1-6H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50241461

(3,7-bis(dimethylamino)phenothiazin-5-ium chloride ...)Show SMILES CN(C)c1ccc2nc3ccc(cc3sc2c1)=[N+](C)C |(17.73,-40.24,;19.06,-41.01,;19.06,-42.55,;20.39,-40.24,;20.4,-38.7,;21.73,-37.93,;23.06,-38.69,;24.39,-37.91,;25.73,-38.69,;27.06,-37.93,;28.39,-38.69,;28.39,-40.23,;27.06,-41,;25.73,-40.23,;24.39,-41.01,;23.06,-40.24,;21.73,-41.01,;29.73,-41,;29.73,-42.54,;31.06,-40.23,)| Show InChI InChI=1S/C16H18N3S/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13/h5-10H,1-4H3/q+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Biochemie-Zentrum der Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TrxR1 at pH 7.4 |
Antimicrob Agents Chemother 52: 183-91 (2007)
Article DOI: 10.1128/AAC.00773-07
BindingDB Entry DOI: 10.7270/Q21V5F0T |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182126
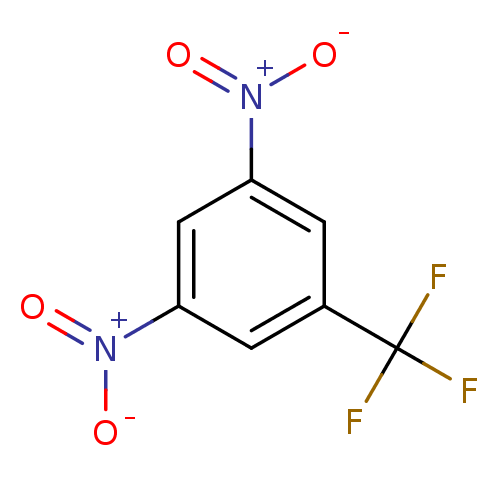
(1,3-dinitro-5-(trifluoromethyl)benzene | CHEMBL380...)Show SMILES [O-][N+](=O)c1cc(cc(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C7H3F3N2O4/c8-7(9,10)4-1-5(11(13)14)3-6(2-4)12(15)16/h1-3H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Plasmodium falciparum (isolate 3D7)) | BDBM50182127

(6-nitroquinoxaline | CHEMBL380630 | TCMDC-123943)Show InChI InChI=1S/C8H5N3O2/c12-11(13)6-1-2-7-8(5-6)10-4-3-9-7/h1-5H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50182128

(4-nitrobenzo[c][1,2,5]thiadiazole | CHEMBL383084)Show InChI InChI=1S/C6H3N3O2S/c10-9(11)5-3-1-2-4-6(5)8-12-7-4/h1-3H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50182121

(4,5-dinitrobenzo[d][1,3]dioxole | CHEMBL206829)Show InChI InChI=1S/C7H4N2O6/c10-8(11)4-1-2-5-7(15-3-14-5)6(4)9(12)13/h1-2H,3H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50182129

(5-nitrobenzo[c][1,2,5]thiadiazole | CHEMBL206713)Show InChI InChI=1S/C6H3N3O2S/c10-9(11)4-1-2-5-6(3-4)8-12-7-5/h1-3H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50068343

(2-Chloro-1,3-dinitro-5-trifluoromethyl-benzene | 2...)Show SMILES [O-][N+](=O)c1cc(cc(c1Cl)[N+]([O-])=O)C(F)(F)F Show InChI InChI=1S/C7H2ClF3N2O4/c8-6-4(12(14)15)1-3(7(9,10)11)2-5(6)13(16)17/h1-2H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50182124

(6,7-dinitroquinoxaline | CHEMBL380953)Show InChI InChI=1S/C8H4N4O4/c13-11(14)7-3-5-6(10-2-1-9-5)4-8(7)12(15)16/h1-4H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50182125

(CHEMBL204687 | bis(2-nitro-4-(trifluoromethyl)phen...)Show SMILES [O-][N+](=O)c1cc(ccc1Sc1ccc(cc1[N+]([O-])=O)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C14H6F6N2O4S/c15-13(16,17)7-1-3-11(9(5-7)21(23)24)27-12-4-2-8(14(18,19)20)6-10(12)22(25)26/h1-6H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50182127

(6-nitroquinoxaline | CHEMBL380630 | TCMDC-123943)Show InChI InChI=1S/C8H5N3O2/c12-11(13)6-1-2-7-8(5-6)10-4-3-9-7/h1-5H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50182126

(1,3-dinitro-5-(trifluoromethyl)benzene | CHEMBL380...)Show SMILES [O-][N+](=O)c1cc(cc(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C7H3F3N2O4/c8-7(9,10)4-1-5(11(13)14)3-6(2-4)12(15)16/h1-3H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic/2, mitochondrial/3
(Homo sapiens (Human)) | BDBM50023678
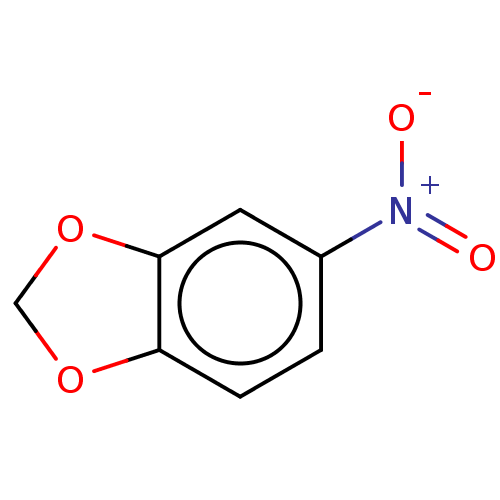
(5-Nitrobenzo[D][1,3]Dioxole | CHEMBL379408)Show InChI InChI=1S/C7H5NO4/c9-8(10)5-1-2-6-7(3-5)12-4-11-6/h1-3H,4H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human TrxR |
Bioorg Med Chem Lett 16: 2283-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.027
BindingDB Entry DOI: 10.7270/Q2FB52HJ |
More data for this
Ligand-Target Pair | |
Dihydrolipoyl dehydrogenase
(Plasmodium falciparum) | BDBM50241461

(3,7-bis(dimethylamino)phenothiazin-5-ium chloride ...)Show SMILES CN(C)c1ccc2nc3ccc(cc3sc2c1)=[N+](C)C |(17.73,-40.24,;19.06,-41.01,;19.06,-42.55,;20.39,-40.24,;20.4,-38.7,;21.73,-37.93,;23.06,-38.69,;24.39,-37.91,;25.73,-38.69,;27.06,-37.93,;28.39,-38.69,;28.39,-40.23,;27.06,-41,;25.73,-40.23,;24.39,-41.01,;23.06,-40.24,;21.73,-41.01,;29.73,-41,;29.73,-42.54,;31.06,-40.23,)| Show InChI InChI=1S/C16H18N3S/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13/h5-10H,1-4H3/q+1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | 7.3 | n/a |
Biochemie-Zentrum der Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant dihydrolipoamide dehydrogenase at pH 7.3 |
Antimicrob Agents Chemother 52: 183-91 (2007)
Article DOI: 10.1128/AAC.00773-07
BindingDB Entry DOI: 10.7270/Q21V5F0T |
More data for this
Ligand-Target Pair | |
Dihydrolipoyl dehydrogenase, mitochondrial
(Sus scrofa) | BDBM50241461

(3,7-bis(dimethylamino)phenothiazin-5-ium chloride ...)Show SMILES CN(C)c1ccc2nc3ccc(cc3sc2c1)=[N+](C)C |(17.73,-40.24,;19.06,-41.01,;19.06,-42.55,;20.39,-40.24,;20.4,-38.7,;21.73,-37.93,;23.06,-38.69,;24.39,-37.91,;25.73,-38.69,;27.06,-37.93,;28.39,-38.69,;28.39,-40.23,;27.06,-41,;25.73,-40.23,;24.39,-41.01,;23.06,-40.24,;21.73,-41.01,;29.73,-41,;29.73,-42.54,;31.06,-40.23,)| Show InChI InChI=1S/C16H18N3S/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13/h5-10H,1-4H3/q+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | 7.3 | n/a |
Biochemie-Zentrum der Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibition of pig recombinant dihydrolipoamide dehydrogenase at pH 7.3 |
Antimicrob Agents Chemother 52: 183-91 (2007)
Article DOI: 10.1128/AAC.00773-07
BindingDB Entry DOI: 10.7270/Q21V5F0T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data