 Found 71 hits with Last Name = 'taylor' and Initial = 'mc'
Found 71 hits with Last Name = 'taylor' and Initial = 'mc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10592

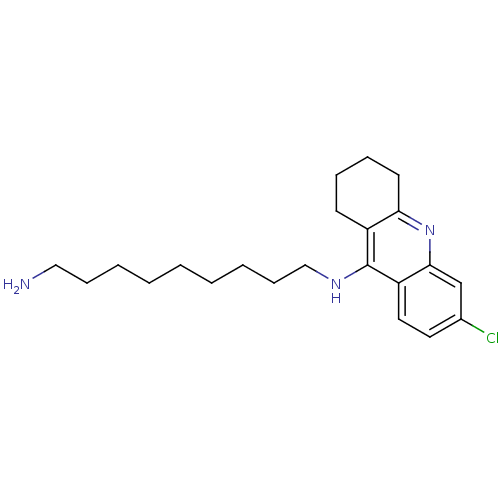
(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman method |
Bioorg Med Chem Lett 24: 5435-8 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.025
BindingDB Entry DOI: 10.7270/Q2G162DS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50108994

(CHEMBL3600555)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 |t:1| Show InChI InChI=1S/C40H49ClN4/c1-27-22-28-24-29(23-27)38-37(25-28)45-35-17-11-9-15-32(35)40(38)43-21-13-7-5-3-2-4-6-12-20-42-39-31-14-8-10-16-34(31)44-36-26-30(41)18-19-33(36)39/h9,11,15,17-19,22,26,28-29H,2-8,10,12-14,16,20-21,23-25H2,1H3,(H,42,44)(H,43,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... |
Bioorg Med Chem 23: 5156-67 (2015)
Article DOI: 10.1016/j.bmc.2015.01.031
BindingDB Entry DOI: 10.7270/Q2XS5X57 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50108990

(CHEMBL3600551)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 |t:1| Show InChI InChI=1S/C42H53ClN4/c1-29-24-30-26-31(25-29)40-39(27-30)47-38-28-32(43)20-21-35(38)42(40)45-23-15-9-7-5-3-2-4-6-8-14-22-44-41-33-16-10-12-18-36(33)46-37-19-13-11-17-34(37)41/h10,12,16,18,20-21,24,28,30-31H,2-9,11,13-15,17,19,22-23,25-27H2,1H3,(H,44,46)(H,45,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... |
Bioorg Med Chem 23: 5156-67 (2015)
Article DOI: 10.1016/j.bmc.2015.01.031
BindingDB Entry DOI: 10.7270/Q2XS5X57 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50108991

(CHEMBL3600552)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 |t:1| Show InChI InChI=1S/C42H52Cl2N4/c1-28-22-29-24-30(23-28)40-39(25-29)48-38-27-32(44)17-19-35(38)42(40)46-21-13-9-7-5-3-2-4-6-8-12-20-45-41-33-14-10-11-15-36(33)47-37-26-31(43)16-18-34(37)41/h16-19,22,26-27,29-30H,2-15,20-21,23-25H2,1H3,(H,45,47)(H,46,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... |
Bioorg Med Chem 23: 5156-67 (2015)
Article DOI: 10.1016/j.bmc.2015.01.031
BindingDB Entry DOI: 10.7270/Q2XS5X57 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50108995

(CHEMBL3600556)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1NCCCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 |t:1| Show InChI InChI=1S/C42H53ClN4/c1-29-24-30-26-31(25-29)40-39(27-30)47-37-19-13-11-17-34(37)42(40)45-23-15-9-7-5-3-2-4-6-8-14-22-44-41-33-16-10-12-18-36(33)46-38-28-32(43)20-21-35(38)41/h11,13,17,19-21,24,28,30-31H,2-10,12,14-16,18,22-23,25-27H2,1H3,(H,44,46)(H,45,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... |
Bioorg Med Chem 23: 5156-67 (2015)
Article DOI: 10.1016/j.bmc.2015.01.031
BindingDB Entry DOI: 10.7270/Q2XS5X57 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50108992
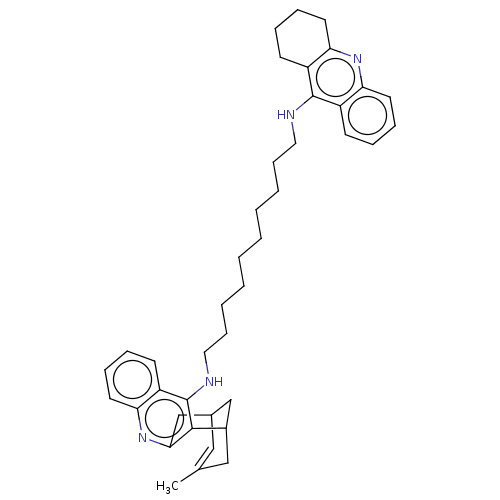
(CHEMBL3600553)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 |t:1| Show InChI InChI=1S/C40H50N4/c1-28-24-29-26-30(25-28)38-37(27-29)44-36-21-13-10-18-33(36)40(38)42-23-15-7-5-3-2-4-6-14-22-41-39-31-16-8-11-19-34(31)43-35-20-12-9-17-32(35)39/h8,10-11,13,16,18-19,21,24,29-30H,2-7,9,12,14-15,17,20,22-23,25-27H2,1H3,(H,41,43)(H,42,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... |
Bioorg Med Chem 23: 5156-67 (2015)
Article DOI: 10.1016/j.bmc.2015.01.031
BindingDB Entry DOI: 10.7270/Q2XS5X57 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194809

(CHEMBL3910753)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCCC#N)c3[C@]([H])(CC(C)=C1)C2 |r,c:32,TLB:12:25:32:31.29.28,30:29:25.3.2:32| Show InChI InChI=1S/C27H34ClN3/c1-19-14-20-16-21(15-19)26-25(17-20)31-24-18-22(28)10-11-23(24)27(26)30-13-9-7-5-3-2-4-6-8-12-29/h10-11,14,18,20-21H,2-9,13,15-17H2,1H3,(H,30,31)/t20-,21+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50108993
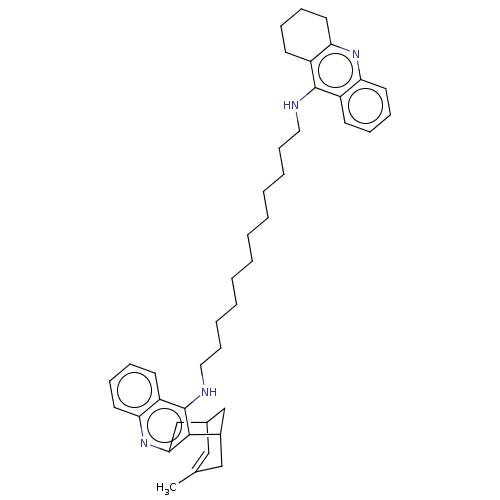
(CHEMBL3600554)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1NCCCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 |t:1| Show InChI InChI=1S/C42H54N4/c1-30-26-31-28-32(27-30)40-39(29-31)46-38-23-15-12-20-35(38)42(40)44-25-17-9-7-5-3-2-4-6-8-16-24-43-41-33-18-10-13-21-36(33)45-37-22-14-11-19-34(37)41/h10,12-13,15,18,20-21,23,26,31-32H,2-9,11,14,16-17,19,22,24-25,27-29H2,1H3,(H,43,45)(H,44,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... |
Bioorg Med Chem 23: 5156-67 (2015)
Article DOI: 10.1016/j.bmc.2015.01.031
BindingDB Entry DOI: 10.7270/Q2XS5X57 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484769

(CHEMBL1958429)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)Nc1ccc(OC(F)F)cc1 Show InChI InChI=1S/C16H14F2N2O5S/c17-16(18)25-13-7-5-12(6-8-13)20-26(23,24)14-3-1-2-11(10-14)4-9-15(21)19-22/h1-10,16,20,22H,(H,19,21)/b9-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194801

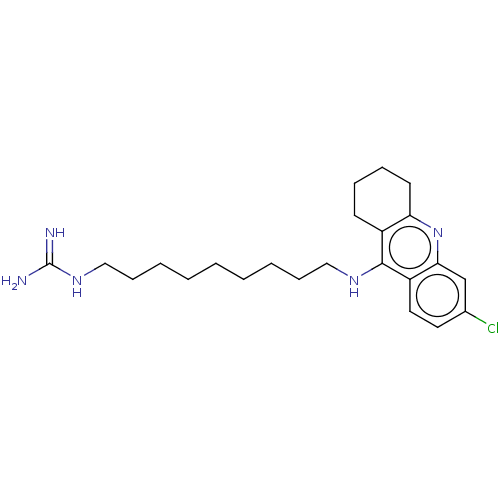
(CHEMBL3910120)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCCNC(N)=N)c3[C@]([H])(CC(C)=C1)C2 |r,c:34,TLB:12:27:34:33.31.30,32:31:27.3.2:34| Show InChI InChI=1S/C27H38ClN5/c1-18-13-19-15-20(14-18)25-24(16-19)33-23-17-21(28)9-10-22(23)26(25)31-11-7-5-3-2-4-6-8-12-32-27(29)30/h9-10,13,17,19-20H,2-8,11-12,14-16H2,1H3,(H,31,33)(H4,29,30,32)/t19-,20+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194800
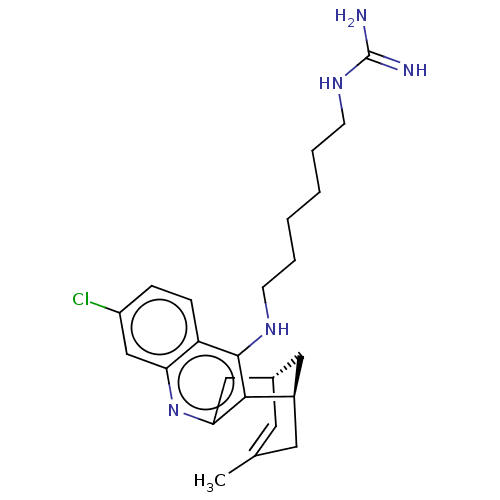
(CHEMBL3890983)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCNC(N)=N)c3[C@]([H])(CC(C)=C1)C2 |r,c:31,TLB:12:24:31:30.28.27,29:28:24.3.2:31| Show InChI InChI=1S/C24H32ClN5/c1-15-10-16-12-17(11-15)22-21(13-16)30-20-14-18(25)6-7-19(20)23(22)28-8-4-2-3-5-9-29-24(26)27/h6-7,10,14,16-17H,2-5,8-9,11-13H2,1H3,(H,28,30)(H4,26,27,29)/t16-,17+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484758
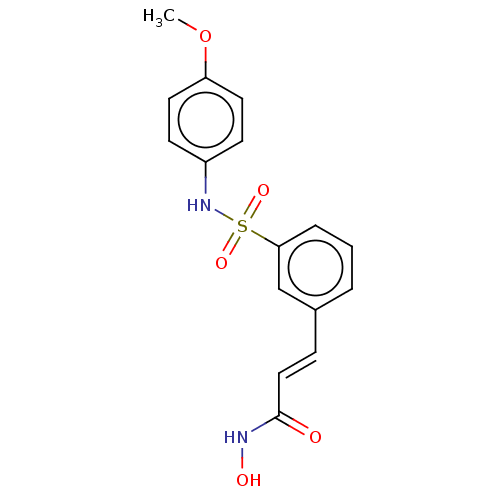
(CHEMBL1958439)Show SMILES COc1ccc(NS(=O)(=O)c2cccc(\C=C\C(=O)NO)c2)cc1 Show InChI InChI=1S/C16H16N2O5S/c1-23-14-8-6-13(7-9-14)18-24(21,22)15-4-2-3-12(11-15)5-10-16(19)17-20/h2-11,18,20H,1H3,(H,17,19)/b10-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194807

(CHEMBL3985938)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCN4CCOCC4)c3[C@]([H])(CC(C)=C1)C2 |r,c:31,TLB:12:23:30:29.27.26,28:27:23.3.2:30| Show InChI InChI=1S/C24H30ClN3O/c1-16-11-17-13-18(12-16)23-22(14-17)27-21-15-19(25)3-4-20(21)24(23)26-5-2-6-28-7-9-29-10-8-28/h3-4,11,15,17-18H,2,5-10,12-14H2,1H3,(H,26,27)/t17-,18+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50435291
(CHEMBL2393095)Show InChI InChI=1S/C22H32ClN3/c23-17-12-13-19-21(16-17)26-20-11-7-6-10-18(20)22(19)25-15-9-5-3-1-2-4-8-14-24/h12-13,16H,1-11,14-15,24H2,(H,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194798

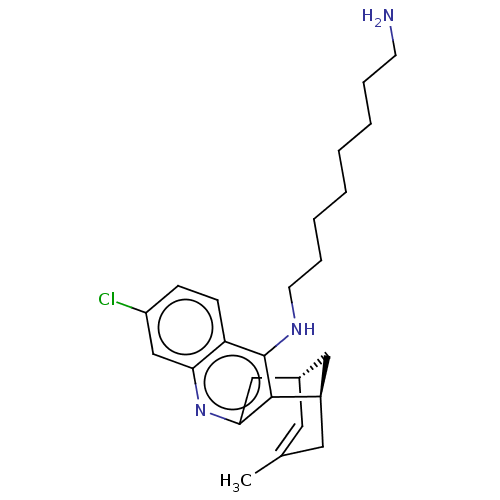
(CHEMBL3901071)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCCN)c3[C@]([H])(CC(C)=C1)C2 |r,c:31,TLB:12:24:31:30.28.27,29:28:24.3.2:31| Show InChI InChI=1S/C26H36ClN3/c1-18-13-19-15-20(14-18)25-24(16-19)30-23-17-21(27)9-10-22(23)26(25)29-12-8-6-4-2-3-5-7-11-28/h9-10,13,17,19-20H,2-8,11-12,14-16,28H2,1H3,(H,29,30)/t19-,20+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM9427
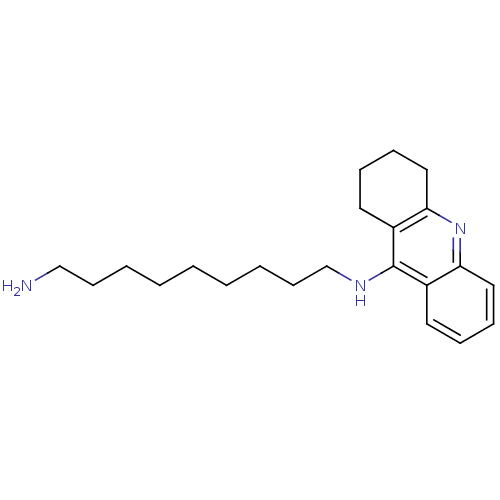
(CHEMBL1083790 | Heterodimeric Tacrine-Based Inhibi...)Show InChI InChI=1S/C22H33N3/c23-16-10-4-2-1-3-5-11-17-24-22-18-12-6-8-14-20(18)25-21-15-9-7-13-19(21)22/h6,8,12,14H,1-5,7,9-11,13,15-17,23H2,(H,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194803
(CHEMBL3906380)Show InChI InChI=1S/C23H34ClN5/c24-17-12-13-19-21(16-17)29-20-11-7-6-10-18(20)22(19)27-14-8-4-2-1-3-5-9-15-28-23(25)26/h12-13,16H,1-11,14-15H2,(H,27,29)(H4,25,26,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194806
(CHEMBL3937637)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCN)c3[C@]([H])(CC(C)=C1)C2 |r,c:30,TLB:12:23:30:29.27.26,28:27:23.3.2:30| Show InChI InChI=1S/C25H34ClN3/c1-17-12-18-14-19(13-17)24-23(15-18)29-22-16-20(26)8-9-21(22)25(24)28-11-7-5-3-2-4-6-10-27/h8-9,12,16,18-19H,2-7,10-11,13-15,27H2,1H3,(H,28,29)/t18-,19+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50036283
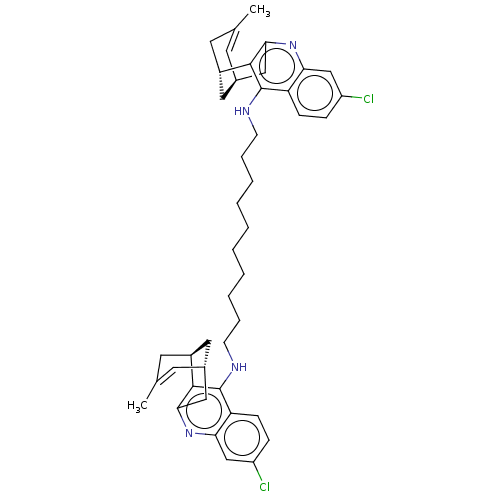
(CHEMBL3353041)Show SMILES [H][C@@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCCCNc4c5c(C[C@@]6([H])C[C@]5([H])CC(C)=C6)nc5cc(Cl)ccc45)c3[C@@]([H])(CC(C)=C1)C2 |r,c:38,57| Show InChI InChI=1S/C44H52Cl2N4/c1-27-17-29-21-31(19-27)41-39(23-29)49-37-25-33(45)11-13-35(37)43(41)47-15-9-7-5-3-4-6-8-10-16-48-44-36-14-12-34(46)26-38(36)50-40-24-30-18-28(2)20-32(22-30)42(40)44/h11-14,17-18,25-26,29-32H,3-10,15-16,19-24H2,1-2H3,(H,47,49)(H,48,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman method |
Bioorg Med Chem Lett 24: 5435-8 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.025
BindingDB Entry DOI: 10.7270/Q2G162DS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194799
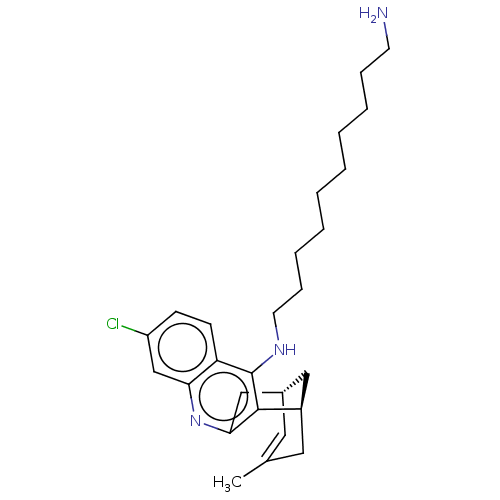
(CHEMBL3972196)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCCCN)c3[C@]([H])(CC(C)=C1)C2 |r,c:32,TLB:12:25:32:31.29.28,30:29:25.3.2:32| Show InChI InChI=1S/C27H38ClN3/c1-19-14-20-16-21(15-19)26-25(17-20)31-24-18-22(28)10-11-23(24)27(26)30-13-9-7-5-3-2-4-6-8-12-29/h10-11,14,18,20-21H,2-9,12-13,15-17,29H2,1H3,(H,30,31)/t20-,21+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484764

(CHEMBL453755)Show InChI InChI=1S/C15H20N2O3/c18-14(11-10-13-7-3-1-4-8-13)16-12-6-2-5-9-15(19)17-20/h1,3-4,7-8,10-11,20H,2,5-6,9,12H2,(H,16,18)(H,17,19)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484757

(CHEMBL1958436)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)Nc1cccc(F)c1 Show InChI InChI=1S/C15H13FN2O4S/c16-12-4-2-5-13(10-12)18-23(21,22)14-6-1-3-11(9-14)7-8-15(19)17-20/h1-10,18,20H,(H,17,19)/b8-7+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484755
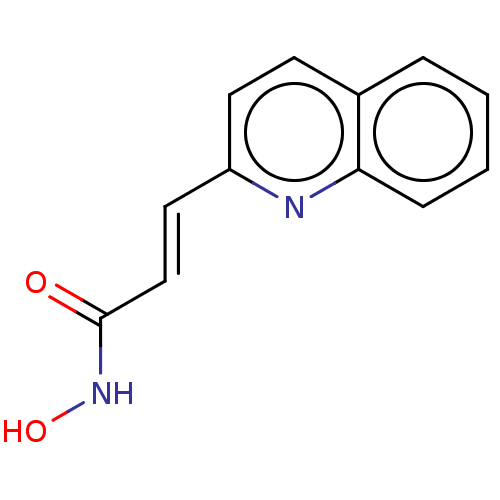
(CHEMBL1958437)Show InChI InChI=1S/C12H10N2O2/c15-12(14-16)8-7-10-6-5-9-3-1-2-4-11(9)13-10/h1-8,16H,(H,14,15)/b8-7+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484761
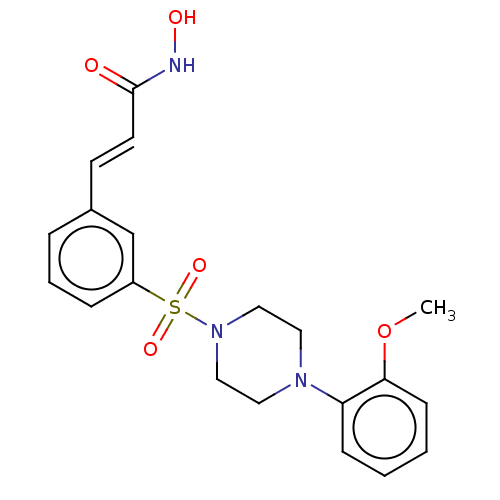
(CHEMBL1958433)Show SMILES COc1ccccc1N1CCN(CC1)S(=O)(=O)c1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C20H23N3O5S/c1-28-19-8-3-2-7-18(19)22-11-13-23(14-12-22)29(26,27)17-6-4-5-16(15-17)9-10-20(24)21-25/h2-10,15,25H,11-14H2,1H3,(H,21,24)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484756

(CHEMBL1958434)Show SMILES COc1cccc(NS(=O)(=O)c2cccc(\C=C\C(=O)NO)c2)c1 Show InChI InChI=1S/C16H16N2O5S/c1-23-14-6-3-5-13(11-14)18-24(21,22)15-7-2-4-12(10-15)8-9-16(19)17-20/h2-11,18,20H,1H3,(H,17,19)/b9-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484762
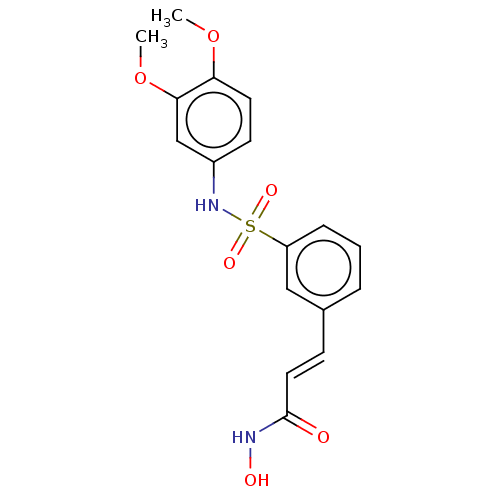
(CHEMBL272847)Show SMILES COc1ccc(NS(=O)(=O)c2cccc(\C=C\C(=O)NO)c2)cc1OC Show InChI InChI=1S/C17H18N2O6S/c1-24-15-8-7-13(11-16(15)25-2)19-26(22,23)14-5-3-4-12(10-14)6-9-17(20)18-21/h3-11,19,21H,1-2H3,(H,18,20)/b9-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM9425
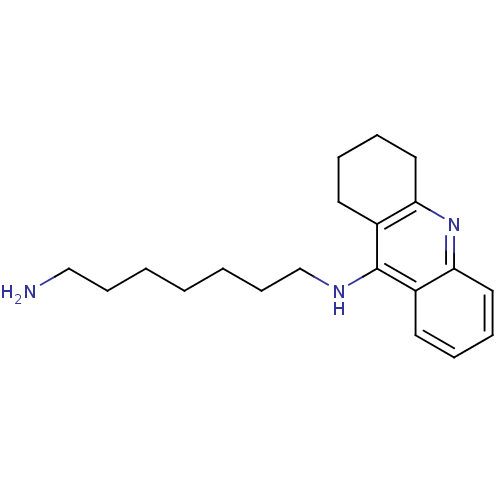
(CHEMBL1083789 | Heterodimeric Tacrine-Based Inhibi...)Show InChI InChI=1S/C20H29N3/c21-14-8-2-1-3-9-15-22-20-16-10-4-6-12-18(16)23-19-13-7-5-11-17(19)20/h4,6,10,12H,1-3,5,7-9,11,13-15,21H2,(H,22,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194808
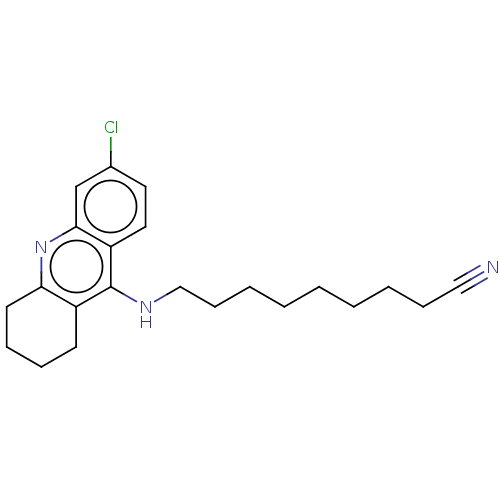
(CHEMBL3902953)Show InChI InChI=1S/C22H28ClN3/c23-17-12-13-19-21(16-17)26-20-11-7-6-10-18(20)22(19)25-15-9-5-3-1-2-4-8-14-24/h12-13,16H,1-11,15H2,(H,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194793

(CHEMBL3977576)Show InChI InChI=1S/C21H31N5/c22-21(23)25-15-9-3-1-2-8-14-24-20-16-10-4-6-12-18(16)26-19-13-7-5-11-17(19)20/h4,6,10,12H,1-3,5,7-9,11,13-15H2,(H,24,26)(H4,22,23,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50268042
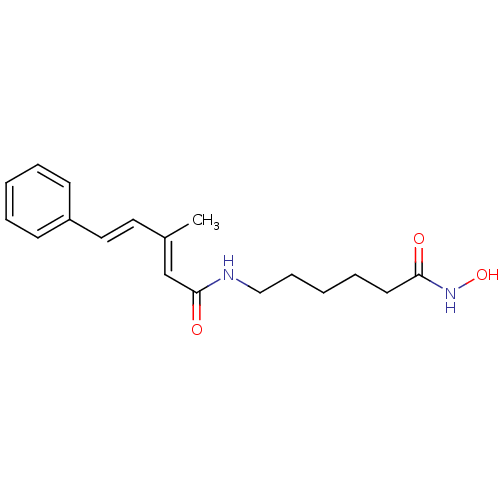
((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194796

(CHEMBL3941365)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCC#N)c3[C@]([H])(CC(C)=C1)C2 |r,c:27,TLB:12:20:27:26.24.23,25:24:20.3.2:27| Show InChI InChI=1S/C22H24ClN3/c1-14-9-15-11-16(10-14)21-20(12-15)26-19-13-17(23)5-6-18(19)22(21)25-8-4-2-3-7-24/h5-6,9,13,15-16H,2-4,8,10-12H2,1H3,(H,25,26)/t15-,16+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484770

(CHEMBL1958430)Show InChI InChI=1S/C13H12N2O3/c1-18-11-5-6-12-9(8-11)2-3-10(14-12)4-7-13(16)15-17/h2-8,17H,1H3,(H,15,16)/b7-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484765

(CHEMBL1955942)Show InChI InChI=1S/C16H20N2O5/c19-15(17-9-3-1-2-4-16(20)18-21)8-6-12-5-7-13-14(10-12)23-11-22-13/h5-8,10,21H,1-4,9,11H2,(H,17,19)(H,18,20)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194790

(CHEMBL3970941)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCC#N)c3[C@]([H])(CC(C)=C1)C2 |r,c:28,TLB:12:21:28:27.25.24,26:25:21.3.2:28| Show InChI InChI=1S/C23H26ClN3/c1-15-10-16-12-17(11-15)22-21(13-16)27-20-14-18(24)6-7-19(20)23(22)26-9-5-3-2-4-8-25/h6-7,10,14,16-17H,2-5,9,11-13H2,1H3,(H,26,27)/t16-,17+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194795

(CHEMBL3945505)Show InChI InChI=1S/C23H35N5/c24-23(25)27-17-11-5-3-1-2-4-10-16-26-22-18-12-6-8-14-20(18)28-21-15-9-7-13-19(21)22/h6,8,12,14H,1-5,7,9-11,13,15-17H2,(H,26,28)(H4,24,25,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194791

(CHEMBL3905154)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCCCCN)c3[C@]([H])(CC(C)=C1)C2 |r,c:33,TLB:12:26:33:32.30.29,31:30:26.3.2:33| Show InChI InChI=1S/C28H40ClN3/c1-20-15-21-17-22(16-20)27-26(18-21)32-25-19-23(29)11-12-24(25)28(27)31-14-10-8-6-4-2-3-5-7-9-13-30/h11-12,15,19,21-22H,2-10,13-14,16-18,30H2,1H3,(H,31,32)/t21-,22+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194797
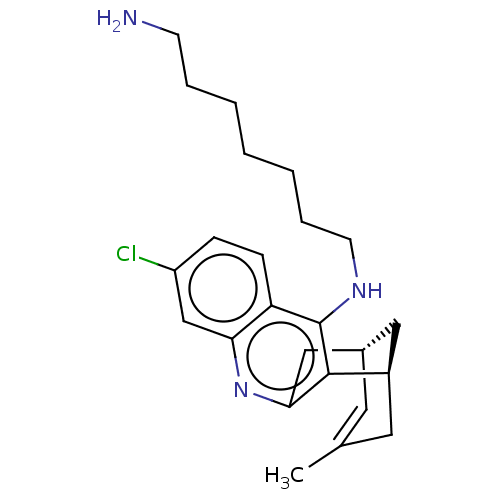
(CHEMBL3939732)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCN)c3[C@]([H])(CC(C)=C1)C2 |r,c:29,TLB:12:22:29:28.26.25,27:26:22.3.2:29| Show InChI InChI=1S/C24H32ClN3/c1-16-11-17-13-18(12-16)23-22(14-17)28-21-15-19(25)7-8-20(21)24(23)27-10-6-4-2-3-5-9-26/h7-8,11,15,17-18H,2-6,9-10,12-14,26H2,1H3,(H,27,28)/t17-,18+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484760
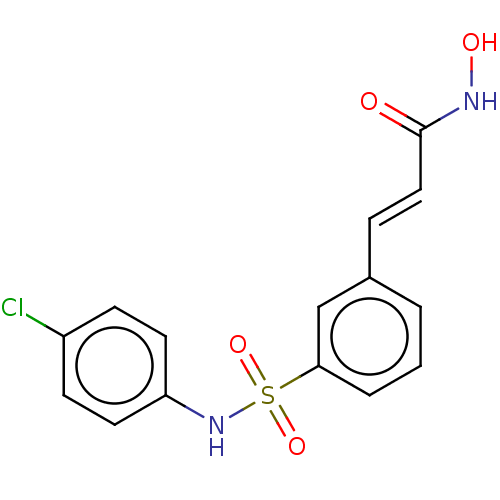
(CHEMBL1958438)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C15H13ClN2O4S/c16-12-5-7-13(8-6-12)18-23(21,22)14-3-1-2-11(10-14)4-9-15(19)17-20/h1-10,18,20H,(H,17,19)/b9-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194804

(CHEMBL3981921)Show InChI InChI=1S/C22H29N3/c23-16-10-4-2-1-3-5-11-17-24-22-18-12-6-8-14-20(18)25-21-15-9-7-13-19(21)22/h6,8,12,14H,1-5,7,9-11,13,15,17H2,(H,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194792
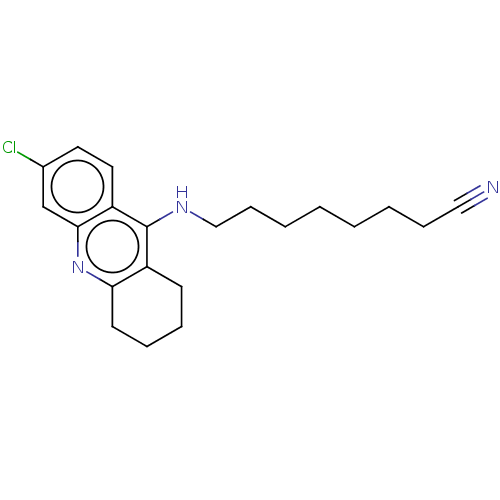
(CHEMBL3973917)Show InChI InChI=1S/C21H26ClN3/c22-16-11-12-18-20(15-16)25-19-10-6-5-9-17(19)21(18)24-14-8-4-2-1-3-7-13-23/h11-12,15H,1-10,14H2,(H,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194802
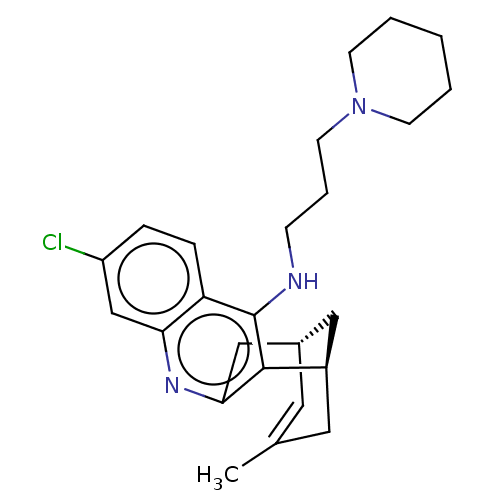
(CHEMBL3908171)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCN4CCCCC4)c3[C@]([H])(CC(C)=C1)C2 |r,c:31,TLB:12:23:30:29.27.26,28:27:23.3.2:30| Show InChI InChI=1S/C25H32ClN3/c1-17-12-18-14-19(13-17)24-23(15-18)28-22-16-20(26)6-7-21(22)25(24)27-8-5-11-29-9-3-2-4-10-29/h6-7,12,16,18-19H,2-5,8-11,13-15H2,1H3,(H,27,28)/t18-,19+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484768

(CHEMBL1958440)Show InChI InChI=1S/C10H8N2O3/c13-9(12-14)5-6-10-11-7-3-1-2-4-8(7)15-10/h1-6,14H,(H,12,13)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194805

(CHEMBL3909021)Show InChI InChI=1S/C20H25N3/c21-14-8-2-1-3-9-15-22-20-16-10-4-6-12-18(16)23-19-13-7-5-11-17(19)20/h4,6,10,12H,1-3,5,7-9,11,13,15H2,(H,22,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484750

(CHEMBL1958428)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)N1CCN(CC1)c1ccccc1Cl Show InChI InChI=1S/C19H20ClN3O4S/c20-17-6-1-2-7-18(17)22-10-12-23(13-11-22)28(26,27)16-5-3-4-15(14-16)8-9-19(24)21-25/h1-9,14,25H,10-13H2,(H,21,24)/b9-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50036282

(CHEMBL3353040)Show SMILES [H][C@@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCNc4c5c(C[C@@]6([H])C[C@]5([H])CC(C)=C6)nc5cc(Cl)ccc45)c3[C@@]([H])(CC(C)=C1)C2 |r,c:36,55| Show InChI InChI=1S/C42H48Cl2N4/c1-25-15-27-19-29(17-25)39-37(21-27)47-35-23-31(43)9-11-33(35)41(39)45-13-7-5-3-4-6-8-14-46-42-34-12-10-32(44)24-36(34)48-38-22-28-16-26(2)18-30(20-28)40(38)42/h9-12,15-16,23-24,27-30H,3-8,13-14,17-22H2,1-2H3,(H,45,47)(H,46,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman method |
Bioorg Med Chem Lett 24: 5435-8 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.025
BindingDB Entry DOI: 10.7270/Q2G162DS |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484751
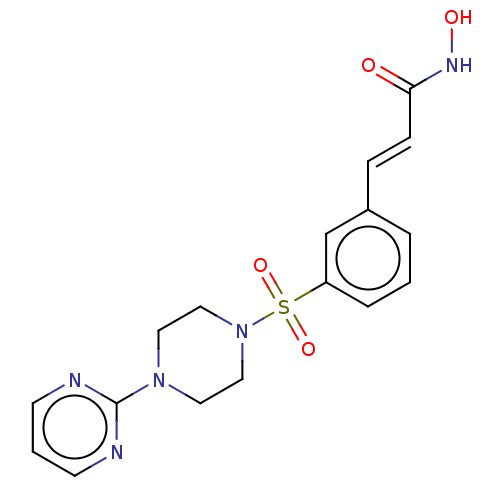
(CHEMBL1958422)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)N1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C17H19N5O4S/c23-16(20-24)6-5-14-3-1-4-15(13-14)27(25,26)22-11-9-21(10-12-22)17-18-7-2-8-19-17/h1-8,13,24H,9-12H2,(H,20,23)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484767

(CHEMBL1958424)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)N1CCN(CC1)c1ccccn1 Show InChI InChI=1S/C18H20N4O4S/c23-18(20-24)8-7-15-4-3-5-16(14-15)27(25,26)22-12-10-21(11-13-22)17-6-1-2-9-19-17/h1-9,14,24H,10-13H2,(H,20,23)/b8-7+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484763
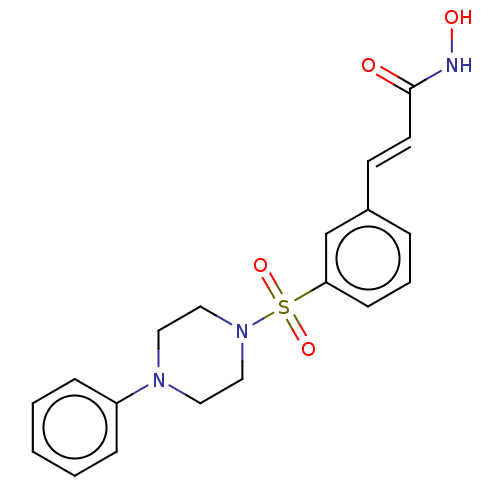
(CHEMBL1958432)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)N1CCN(CC1)c1ccccc1 Show InChI InChI=1S/C19H21N3O4S/c23-19(20-24)10-9-16-5-4-8-18(15-16)27(25,26)22-13-11-21(12-14-22)17-6-2-1-3-7-17/h1-10,15,24H,11-14H2,(H,20,23)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50484759

(CHEMBL1958423)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)N1CCN(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H20ClN3O4S/c20-16-5-7-17(8-6-16)22-10-12-23(13-11-22)28(26,27)18-3-1-2-15(14-18)4-9-19(24)21-25/h1-9,14,25H,10-13H2,(H,21,24)/b9-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
London School of Hygiene and Tropical Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC expressed in human HeLa cells after 1 hr by fluorescence analysis |
Bioorg Med Chem Lett 22: 1886-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.072
BindingDB Entry DOI: 10.7270/Q2QF8WQT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50194794
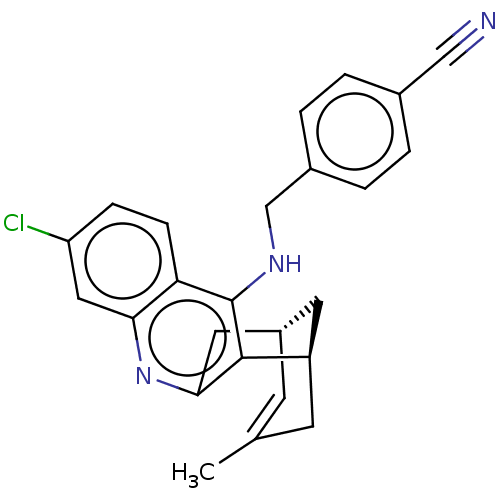
(CHEMBL3981794)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCc4ccc(cc4)C#N)c3[C@]([H])(CC(C)=C1)C2 |r,c:31,TLB:12:23:30:29.27.26,28:27:23.3.2:30| Show InChI InChI=1S/C25H22ClN3/c1-15-8-18-10-19(9-15)24-23(11-18)29-22-12-20(26)6-7-21(22)25(24)28-14-17-4-2-16(13-27)3-5-17/h2-8,12,18-19H,9-11,14H2,1H3,(H,28,29)/t18-,19+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... |
Bioorg Med Chem 24: 5162-5171 (2016)
Article DOI: 10.1016/j.bmc.2016.08.036
BindingDB Entry DOI: 10.7270/Q2V98B0N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data