

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50405790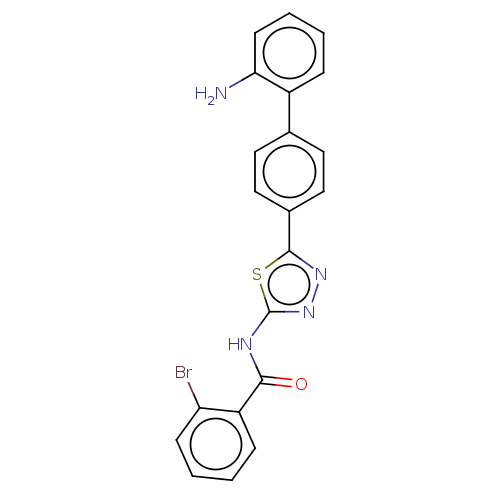 (CHEMBL5284121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist potency against carbachol induced inhibition of electrically stimulated guinea pig atria Muscarinic acetylcholine receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50405760 (CHEMBL5284133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist potency against muscarinic receptors was assed by antagonism of carbachol induced inhibition of electrically stimulated guinea pig atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339570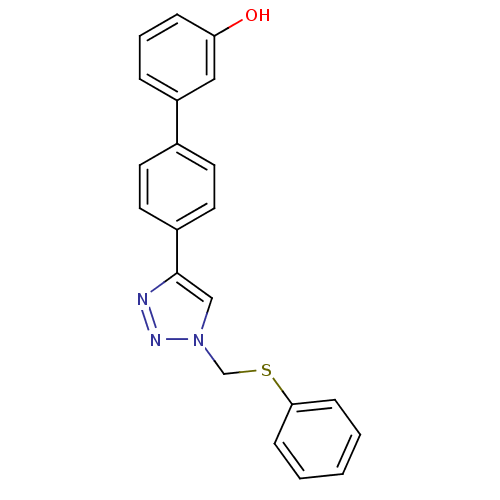 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi del Piemonte Orientale A. Avogadro Curated by ChEMBL | Assay Description Inhibition of HDAC in human SHSY5Y cells by fluorimetric cellular activity assay | J Med Chem 52: 2776-85 (2009) Article DOI: 10.1021/jm801529c BindingDB Entry DOI: 10.7270/Q2D221F1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339571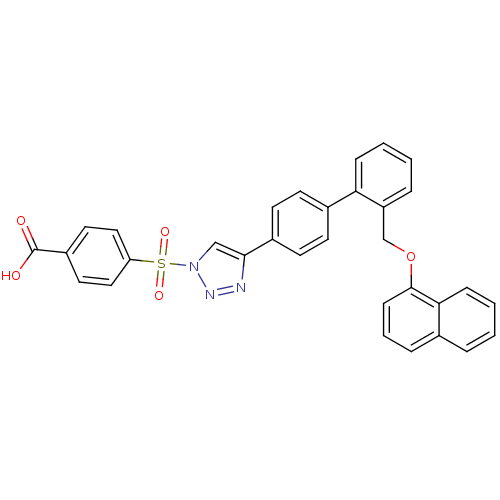 (4-{4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50386165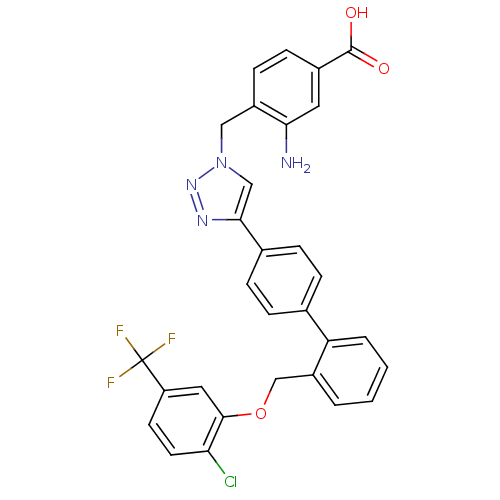 (CHEMBL2042365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell assessed as inhibition of PGE2 production preincubated for 15 mins before substrate addit... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50539779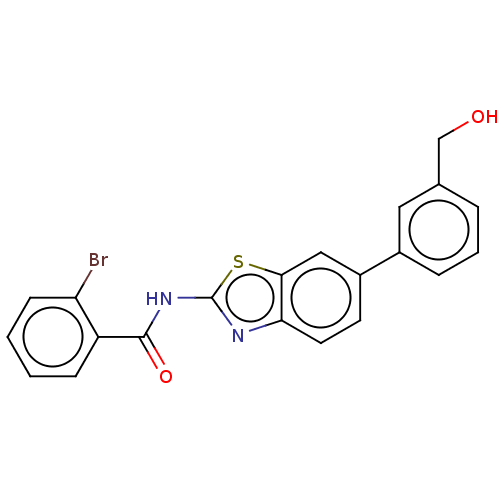 (CHEMBL4647475) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta induced PGE2 release using PGH2 as substrate preincubated for 15 mins foll... | ACS Med Chem Lett 11: 783-789 (2020) Article DOI: 10.1021/acsmedchemlett.9b00618 BindingDB Entry DOI: 10.7270/Q2Z32361 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339571 (4-{4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339572 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386165 (CHEMBL2042365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500813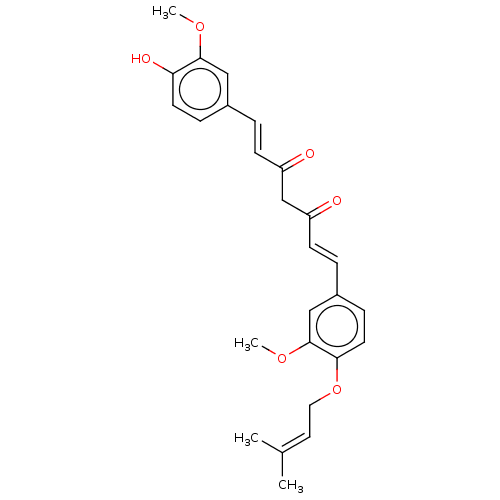 (CHEMBL1087690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50386164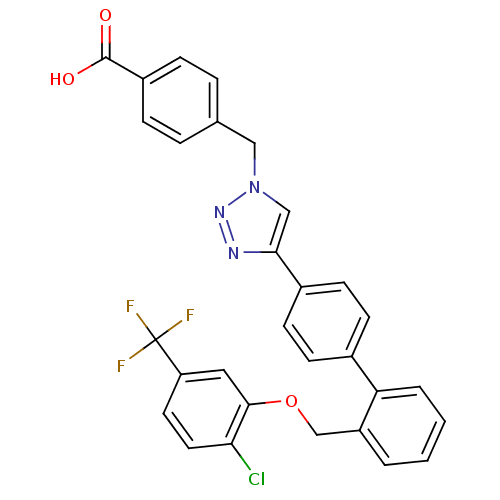 (CHEMBL2042366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell assessed as inhibition of PGE2 production preincubated for 15 mins before substrate addit... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500810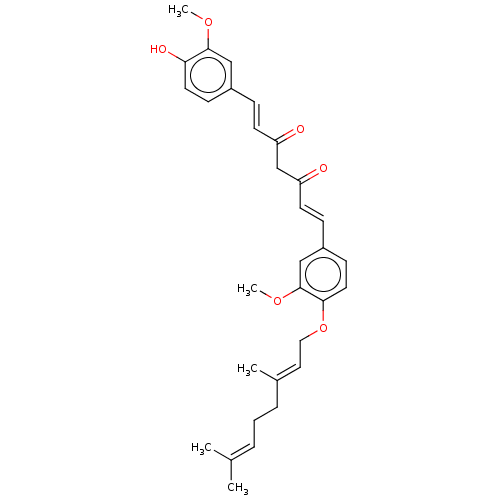 (CHEMBL3758791) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50386154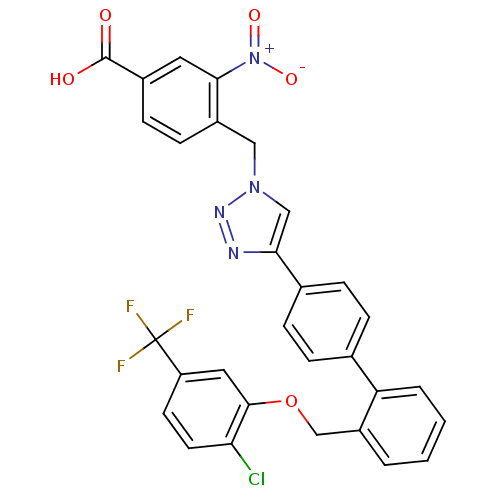 (CHEMBL2042361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell assessed as inhibition of PGE2 production preincubated for 15 mins before substrate addit... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50480332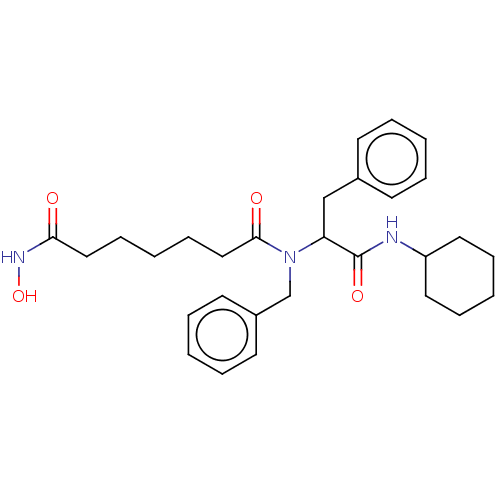 (CHEMBL519853) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi del Piemonte Orientale A. Avogadro Curated by ChEMBL | Assay Description Inhibition of HDAC in human SHSY5Y cells by fluorimetric cellular activity assay | J Med Chem 52: 2776-85 (2009) Article DOI: 10.1021/jm801529c BindingDB Entry DOI: 10.7270/Q2D221F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386163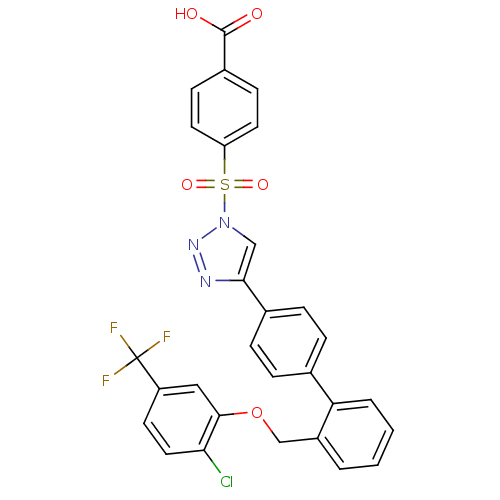 (CHEMBL2042367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophil preincubated for 15 mins before substrate arachidonic acid addition measured after 10 mins by HPLC m... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50539778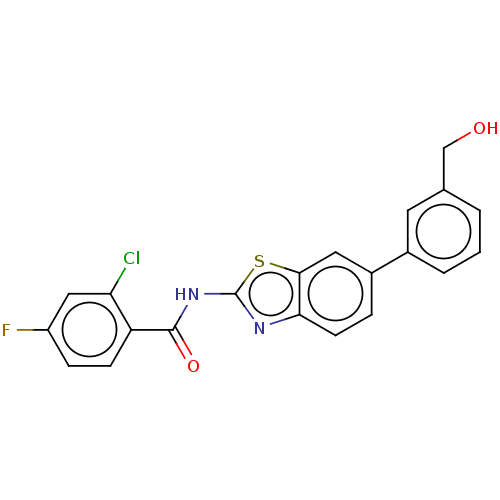 (CHEMBL4636106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta induced PGE2 release using PGH2 as substrate preincubated for 15 mins foll... | ACS Med Chem Lett 11: 783-789 (2020) Article DOI: 10.1021/acsmedchemlett.9b00618 BindingDB Entry DOI: 10.7270/Q2Z32361 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386164 (CHEMBL2042366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50539781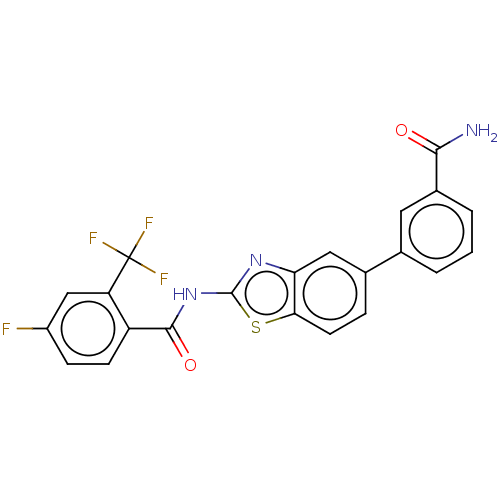 (CHEMBL4647634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta induced PGE2 release using PGH2 as substrate preincubated for 15 mins foll... | ACS Med Chem Lett 11: 783-789 (2020) Article DOI: 10.1021/acsmedchemlett.9b00618 BindingDB Entry DOI: 10.7270/Q2Z32361 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339573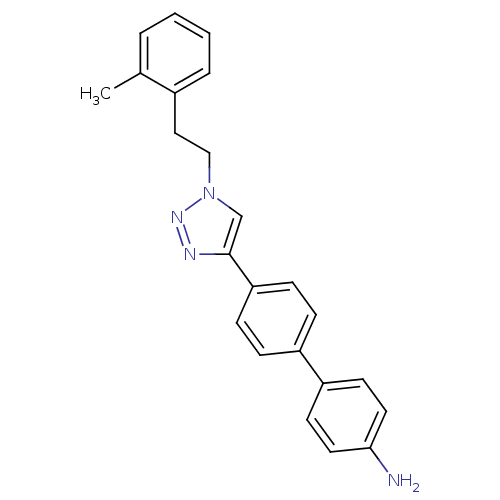 (4'-[1-(2-o-Tolyl-ethyl)-1H-[1,2,3]triazol-4-yl]-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50405791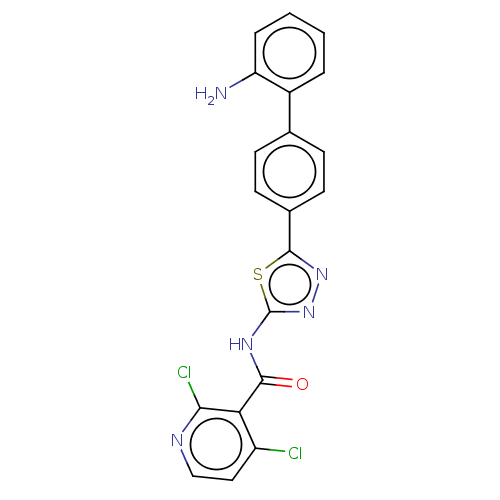 (CHEMBL5285589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist potency against carbachol induced inhibition of electrically stimulated guinea pig atria Muscarinic acetylcholine receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386163 (CHEMBL2042367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50577910 (CHEMBL4866994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in A23187/AA-stimulated human intact neutrophils assessed as reduction in LTB4/5-HETE formation measured after 20 mins by RP-HPLC ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113693 BindingDB Entry DOI: 10.7270/Q2XD15GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500817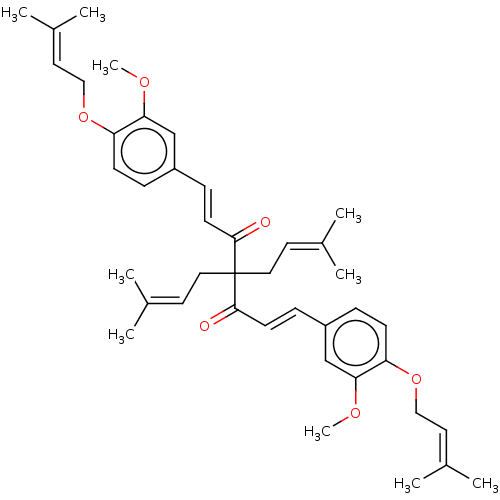 (CHEMBL3758432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500816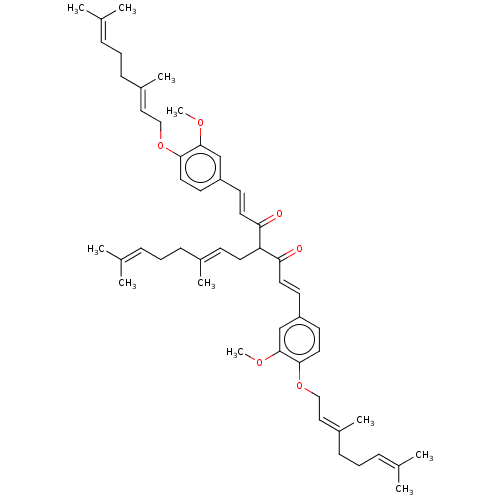 (CHEMBL3758656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500809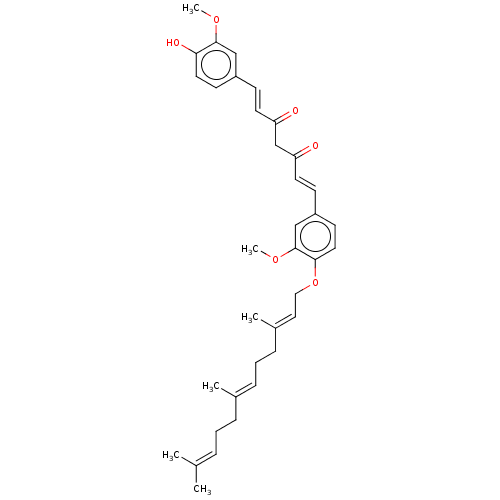 (CHEMBL3759529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500815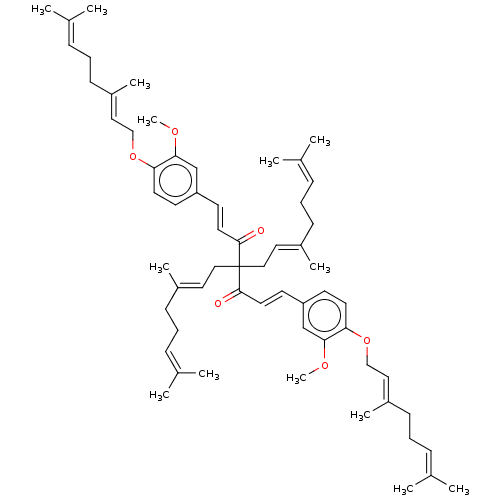 (CHEMBL3759699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500812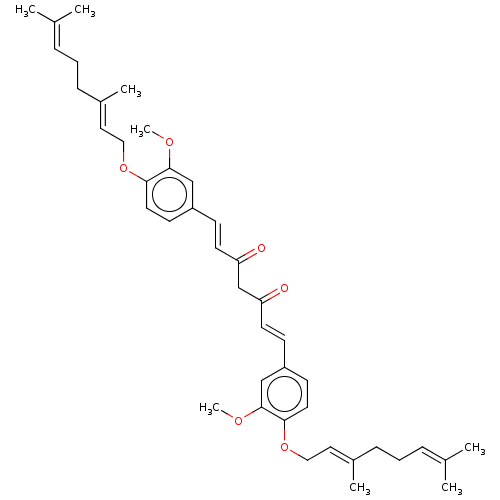 (CHEMBL3758253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500814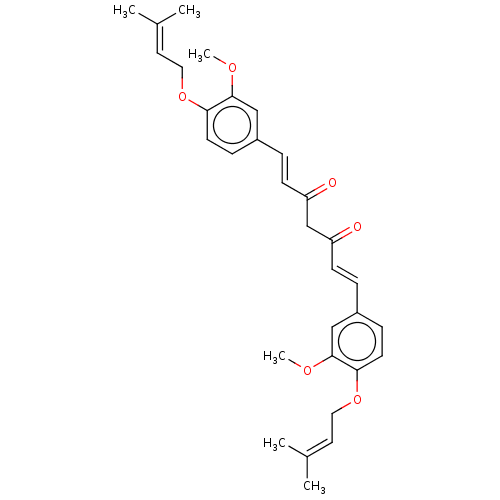 (CHEMBL1087807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500811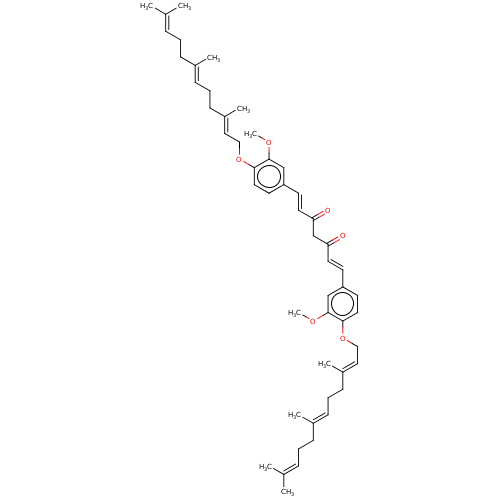 (CHEMBL3759749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386154 (CHEMBL2042361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386164 (CHEMBL2042366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophil preincubated for 15 mins before substrate arachidonic acid addition measured after 10 mins by HPLC m... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500808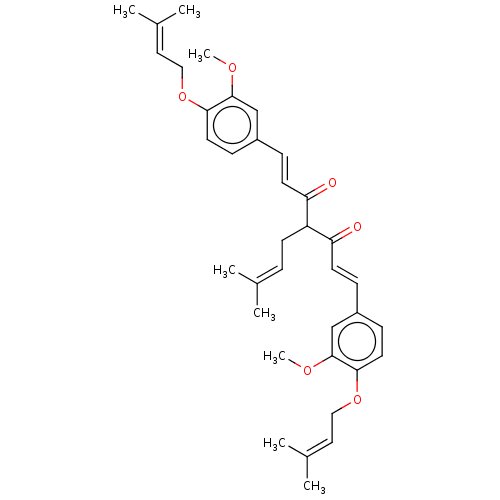 (CHEMBL3758528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386165 (CHEMBL2042365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophil preincubated for 15 mins before substrate arachidonic acid addition measured after 10 mins by HPLC m... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50539780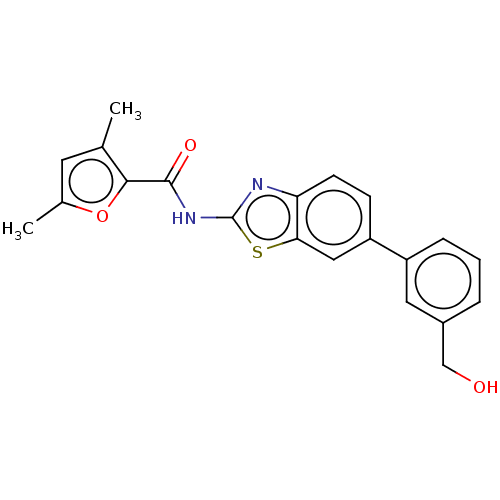 (CHEMBL4643829) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta induced PGE2 release using PGH2 as substrate preincubated for 15 mins foll... | ACS Med Chem Lett 11: 783-789 (2020) Article DOI: 10.1021/acsmedchemlett.9b00618 BindingDB Entry DOI: 10.7270/Q2Z32361 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339574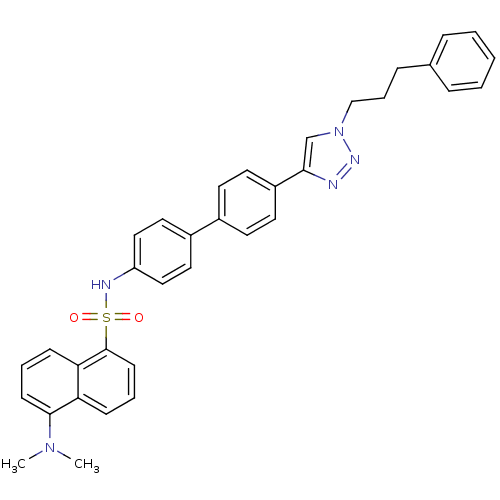 (5-Dimethylamino-naphthalene-1-sulfonic acid{4'-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50405761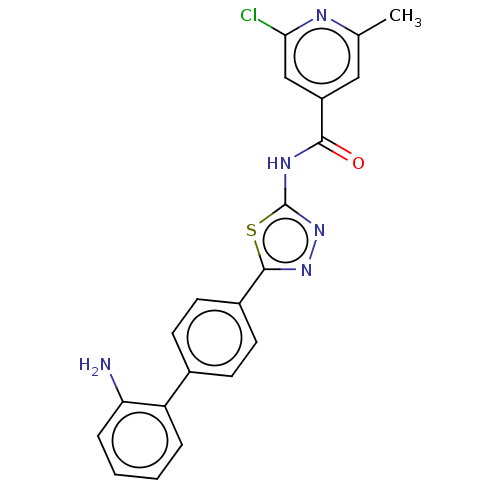 (CHEMBL5274544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist potency against carbachol induced inhibition of electrically stimulated guinea pig atria Muscarinic acetylcholine receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339575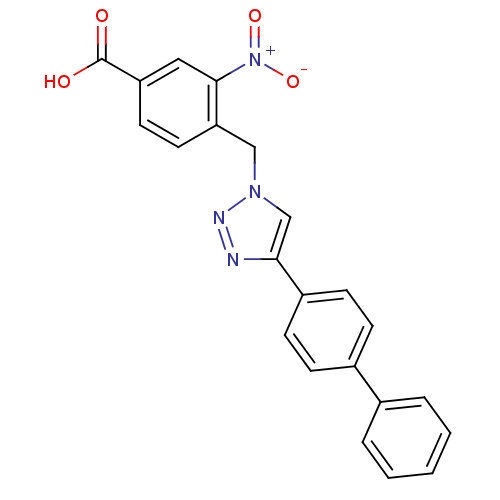 (4-(4-Biphenyl-4-yl-[1,2,3]triazol-1-ylmethyl)-3-ni...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50577910 (CHEMBL4866994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mPGES-1 obtained from IL-beta stimulated human A549 cells microsomes assessed as residual activity by measuring conversion of PGH2 to P... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113693 BindingDB Entry DOI: 10.7270/Q2XD15GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339574 (5-Dimethylamino-naphthalene-1-sulfonic acid{4'-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50480333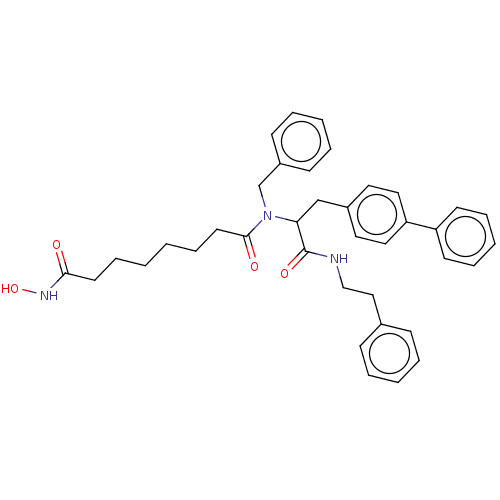 (CHEMBL503268) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi del Piemonte Orientale A. Avogadro Curated by ChEMBL | Assay Description Inhibition of HDAC in human SHSY5Y cells by fluorimetric cellular activity assay | J Med Chem 52: 2776-85 (2009) Article DOI: 10.1021/jm801529c BindingDB Entry DOI: 10.7270/Q2D221F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386166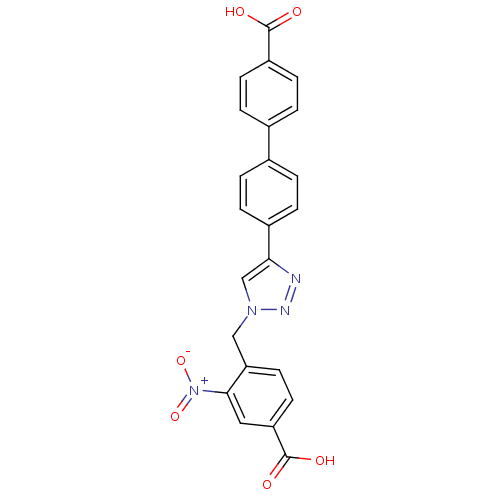 (CHEMBL2042364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophil preincubated for 15 mins before substrate arachidonic acid addition measured after 10 mins by HPLC m... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386166 (CHEMBL2042364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386154 (CHEMBL2042361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophil preincubated for 15 mins before substrate arachidonic acid addition measured after 10 mins by HPLC m... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50386153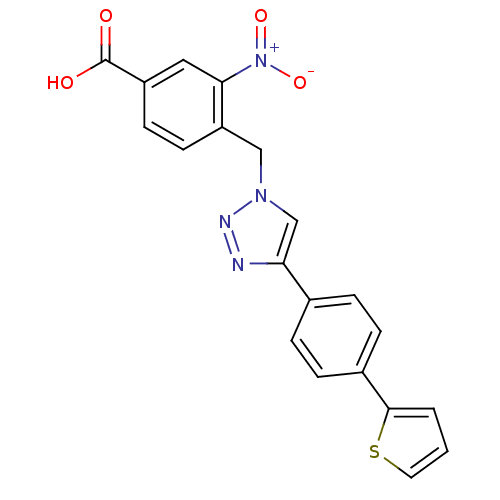 (CHEMBL2042362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell assessed as inhibition of PGE2 production preincubated for 15 mins before substrate addit... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339576 (4-(5-Benzyl-3-phenylsulfanylmethyl-3H-[1,2,3]triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386159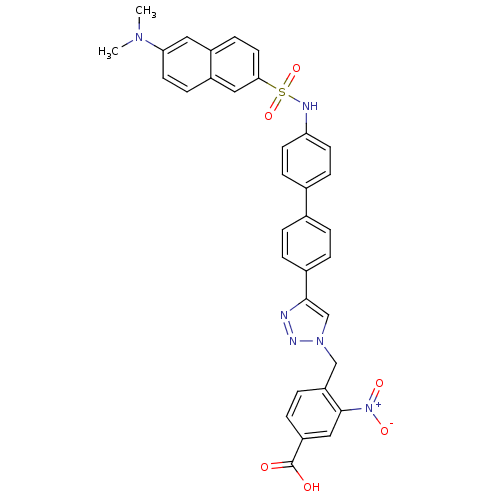 (CHEMBL2042356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339575 (4-(4-Biphenyl-4-yl-[1,2,3]triazol-1-ylmethyl)-3-ni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 178 total ) | Next | Last >> |