 Found 12 hits with Last Name = 'moore' and Initial = 'mj'
Found 12 hits with Last Name = 'moore' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180764
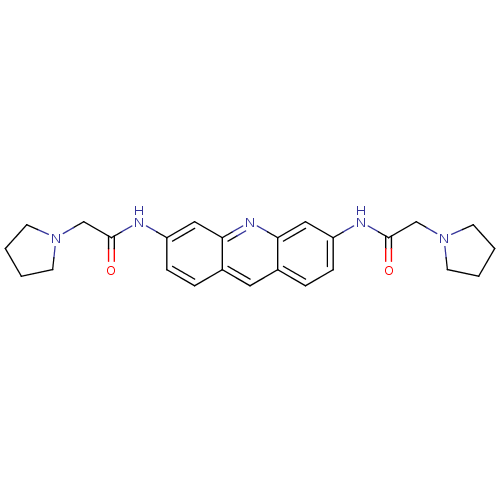
(2-pyrrolidin-1-yl-N-[6-(2-pyrrolidin-1-yl-acetylam...)Show SMILES O=C(CN1CCCC1)Nc1ccc2cc3ccc(NC(=O)CN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C25H29N5O2/c31-24(16-29-9-1-2-10-29)26-20-7-5-18-13-19-6-8-21(15-23(19)28-22(18)14-20)27-25(32)17-30-11-3-4-12-30/h5-8,13-15H,1-4,9-12,16-17H2,(H,26,31)(H,27,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180765
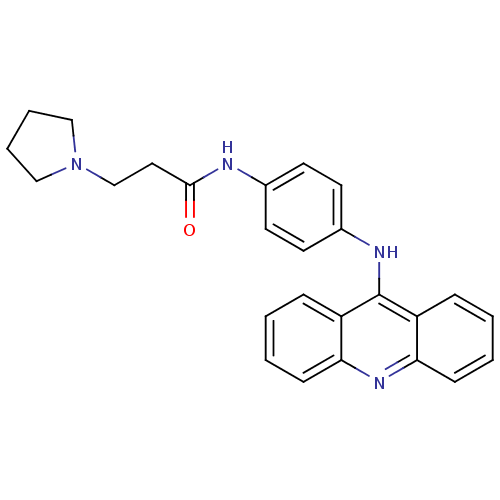
(CHEMBL381966 | N-[4-(acridin-9-ylamino)-phenyl]-3-...)Show SMILES O=C(CCN1CCCC1)Nc1ccc(Nc2c3ccccc3nc3ccccc23)cc1 Show InChI InChI=1S/C26H26N4O/c31-25(15-18-30-16-5-6-17-30)27-19-11-13-20(14-12-19)28-26-21-7-1-3-9-23(21)29-24-10-4-2-8-22(24)26/h1-4,7-14H,5-6,15-18H2,(H,27,31)(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180767
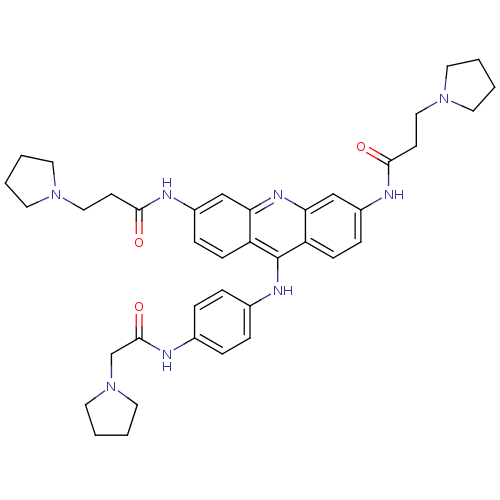
(3,6-bis[3-(pyrrolidin-1-yl)propionamido]-9-{4'-[2'...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2c(Nc3ccc(NC(=O)CN4CCCC4)cc3)c3ccc(NC(=O)CCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C39H48N8O3/c48-36(15-23-45-17-1-2-18-45)41-30-11-13-32-34(25-30)44-35-26-31(42-37(49)16-24-46-19-3-4-20-46)12-14-33(35)39(32)43-29-9-7-28(8-10-29)40-38(50)27-47-21-5-6-22-47/h7-14,25-26H,1-6,15-24,27H2,(H,40,50)(H,41,48)(H,42,49)(H,43,44) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 167 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50134030
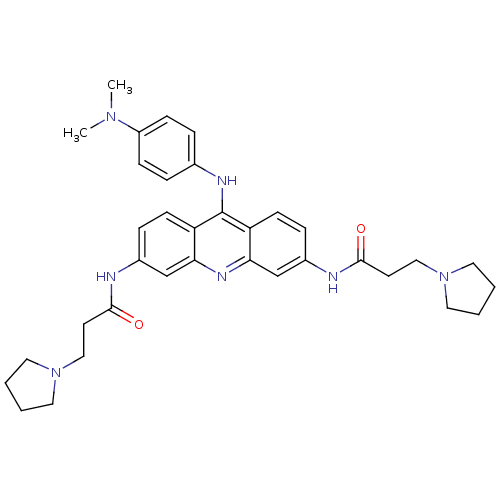
(9-[4-(N,N-dimethylamino)phenylamino]-3,6-bis(3-pyr...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C35H43N7O2/c1-40(2)28-11-7-25(8-12-28)38-35-29-13-9-26(36-33(43)15-21-41-17-3-4-18-41)23-31(29)39-32-24-27(10-14-30(32)35)37-34(44)16-22-42-19-5-6-20-42/h7-14,23-24H,3-6,15-22H2,1-2H3,(H,36,43)(H,37,44)(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180770
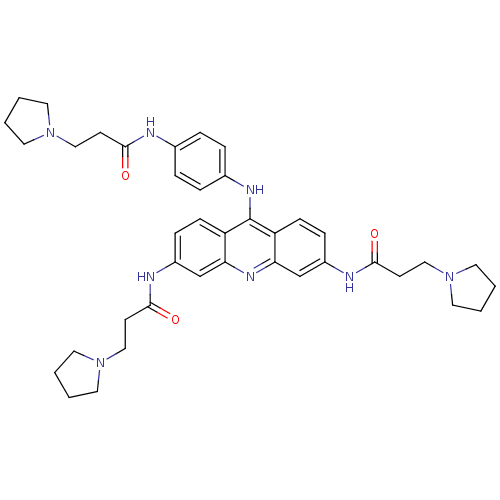
(3,6-bis[3-(pyrrolidin-1-yl)propionamido]-9-{4'-[3'...)Show SMILES O=C(CCN1CCCC1)Nc1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C40H50N8O3/c49-37(15-24-46-18-1-2-19-46)41-29-7-9-30(10-8-29)44-40-33-13-11-31(42-38(50)16-25-47-20-3-4-21-47)27-35(33)45-36-28-32(12-14-34(36)40)43-39(51)17-26-48-22-5-6-23-48/h7-14,27-28H,1-6,15-26H2,(H,41,49)(H,42,50)(H,43,51)(H,44,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180773
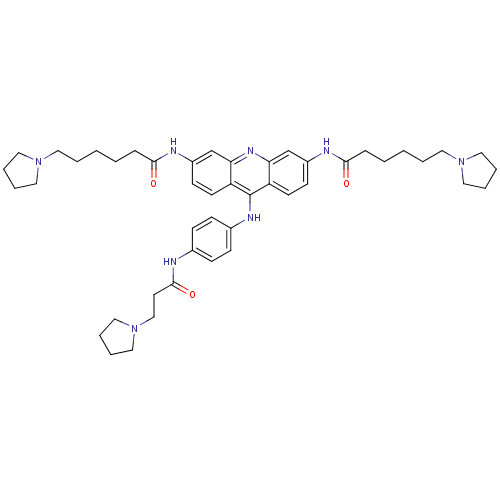
(3,6-bis[6-(pyrrolidin-1-yl)hexanamido]-9-{4'-[3''-...)Show SMILES O=C(CCCCCN1CCCC1)Nc1ccc2c(Nc3ccc(NC(=O)CCN4CCCC4)cc3)c3ccc(NC(=O)CCCCCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C46H62N8O3/c55-43(13-3-1-5-24-52-26-7-8-27-52)48-37-19-21-39-41(33-37)51-42-34-38(49-44(56)14-4-2-6-25-53-28-9-10-29-53)20-22-40(42)46(39)50-36-17-15-35(16-18-36)47-45(57)23-32-54-30-11-12-31-54/h15-22,33-34H,1-14,23-32H2,(H,47,57)(H,48,55)(H,49,56)(H,50,51) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 146 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180766

(3,6-bis[4-(pyrrolidin-1-yl)butanamido]-9-{4'-[3''-...)Show SMILES O=C(CCCN1CCCC1)Nc1ccc2c(Nc3ccc(NC(=O)CCN4CCCC4)cc3)c3ccc(NC(=O)CCCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C42H54N8O3/c51-39(9-7-26-48-20-1-2-21-48)44-33-15-17-35-37(29-33)47-38-30-34(45-40(52)10-8-27-49-22-3-4-23-49)16-18-36(38)42(35)46-32-13-11-31(12-14-32)43-41(53)19-28-50-24-5-6-25-50/h11-18,29-30H,1-10,19-28H2,(H,43,53)(H,44,51)(H,45,52)(H,46,47) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 326 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180771
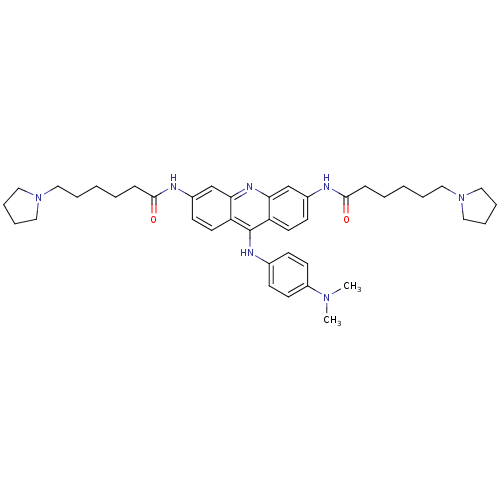
(3,6-bis[6-(pyrrolidin-1-yl)hexanamido]-9-[4'-(N,N-...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCCCCN4CCCC4)cc3nc3cc(NC(=O)CCCCCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C41H55N7O2/c1-46(2)34-19-15-31(16-20-34)44-41-35-21-17-32(42-39(49)13-5-3-7-23-47-25-9-10-26-47)29-37(35)45-38-30-33(18-22-36(38)41)43-40(50)14-6-4-8-24-48-27-11-12-28-48/h15-22,29-30H,3-14,23-28H2,1-2H3,(H,42,49)(H,43,50)(H,44,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.91E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180768
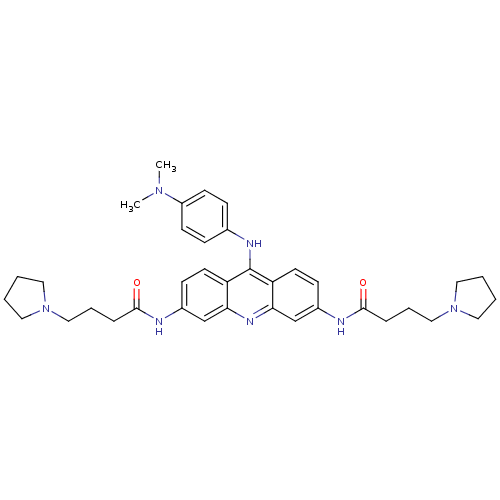
(3,6-[4-(pyrrolidin-1-yl)butanamido]-9-[4'-(N,N-dim...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCCN4CCCC4)cc3nc3cc(NC(=O)CCCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C37H47N7O2/c1-42(2)30-15-11-27(12-16-30)40-37-31-17-13-28(38-35(45)9-7-23-43-19-3-4-20-43)25-33(31)41-34-26-29(14-18-32(34)37)39-36(46)10-8-24-44-21-5-6-22-44/h11-18,25-26H,3-10,19-24H2,1-2H3,(H,38,45)(H,39,46)(H,40,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180769
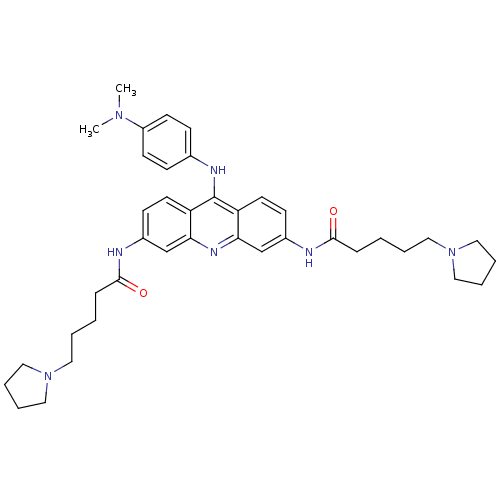
(3,6-bis[5-(pyrrolidin-1-yl)pentanamido]-9-[4'-(N,N...)Show SMILES CN(C)c1ccc(Nc2c3ccc(NC(=O)CCCCN4CCCC4)cc3nc3cc(NC(=O)CCCCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C39H51N7O2/c1-44(2)32-17-13-29(14-18-32)42-39-33-19-15-30(40-37(47)11-3-5-21-45-23-7-8-24-45)27-35(33)43-36-28-31(16-20-34(36)39)41-38(48)12-4-6-22-46-25-9-10-26-46/h13-20,27-28H,3-12,21-26H2,1-2H3,(H,40,47)(H,41,48)(H,42,43) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180772
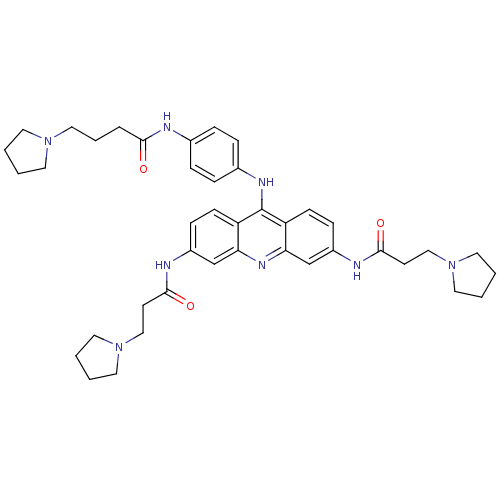
(3,6-bis[3-(pyrrolidin-1-yl)propionamido]-9-{4'-[3'...)Show SMILES O=C(CCCN1CCCC1)Nc1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1 Show InChI InChI=1S/C41H52N8O3/c50-38(8-7-25-47-19-1-2-20-47)42-30-9-11-31(12-10-30)45-41-34-15-13-32(43-39(51)17-26-48-21-3-4-22-48)28-36(34)46-37-29-33(14-16-35(37)41)44-40(52)18-27-49-23-5-6-24-49/h9-16,28-29H,1-8,17-27H2,(H,42,50)(H,43,51)(H,44,52)(H,45,46) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50180763
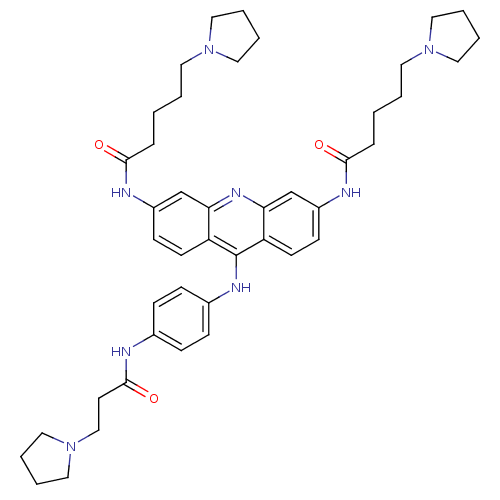
(3,6-bis[5-(pyrrolidin-1-yl)pentanamido]-9-{4'-[3''...)Show SMILES O=C(CCCCN1CCCC1)Nc1ccc2c(Nc3ccc(NC(=O)CCN4CCCC4)cc3)c3ccc(NC(=O)CCCCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C44H58N8O3/c53-41(11-1-3-22-50-24-5-6-25-50)46-35-17-19-37-39(31-35)49-40-32-36(47-42(54)12-2-4-23-51-26-7-8-27-51)18-20-38(40)44(37)48-34-15-13-33(14-16-34)45-43(55)21-30-52-28-9-10-29-52/h13-20,31-32H,1-12,21-30H2,(H,45,55)(H,46,53)(H,47,54)(H,48,49) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 255 | n/a | n/a | n/a | n/a |
University of London
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity from human A2780 cells |
J Med Chem 49: 582-99 (2006)
Article DOI: 10.1021/jm050555a
BindingDB Entry DOI: 10.7270/Q2CN73GR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data