 Found 145 hits with Last Name = 'bodnarchuk' and Initial = 'ms'
Found 145 hits with Last Name = 'bodnarchuk' and Initial = 'ms' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559562

(CHEMBL4762834)Show SMILES Cn1c(CNC(=O)C2(CC(O)=O)Cc3ccccc3C2)nc2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559561
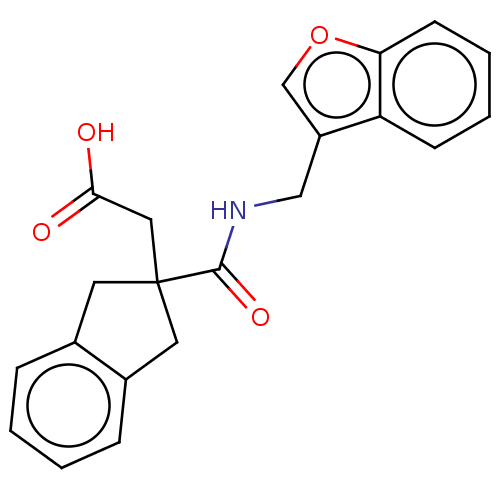
(CHEMBL4760006) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559558

(CHEMBL4764936)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1cc2ccccc2s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559555

(CHEMBL4745988)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1nc2ccccc2s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559556
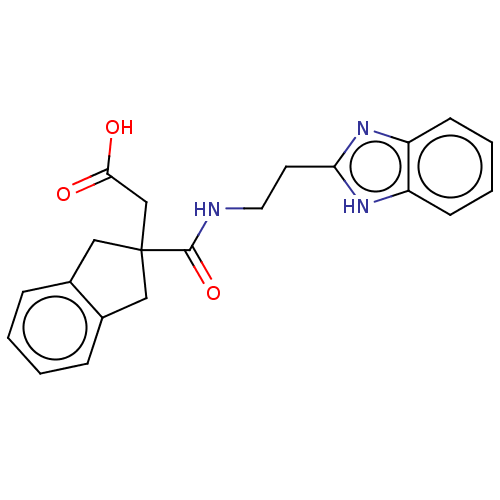
(CHEMBL4749889)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1nc2ccccc2[nH]1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559557

(CHEMBL4794689)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1cc2ccccc2[nH]1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559559
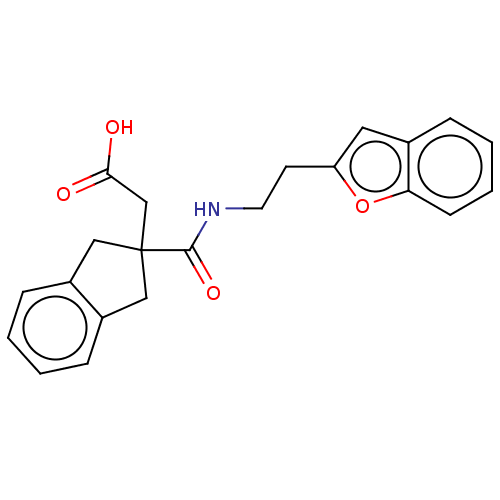
(CHEMBL4746129)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1cc2ccccc2o1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559560
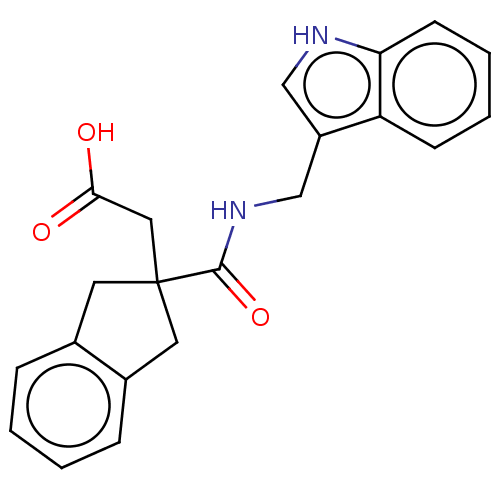
(CHEMBL4756804)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1c[nH]c2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559552

(CHEMBL4776002) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559563

(CHEMBL4776454)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1nc2ccccc2[nH]1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559564
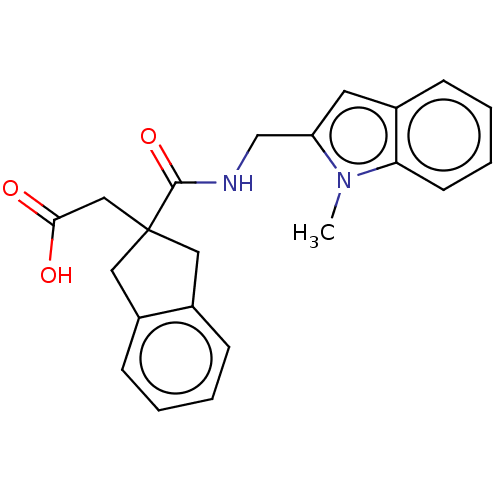
(CHEMBL4746167)Show SMILES Cn1c(CNC(=O)C2(CC(O)=O)Cc3ccccc3C2)cc2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559565
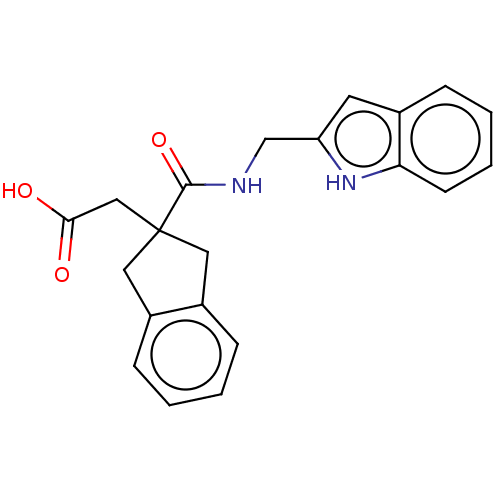
(CHEMBL4776453)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1cc2ccccc2[nH]1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559566

(CHEMBL4749721) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559567
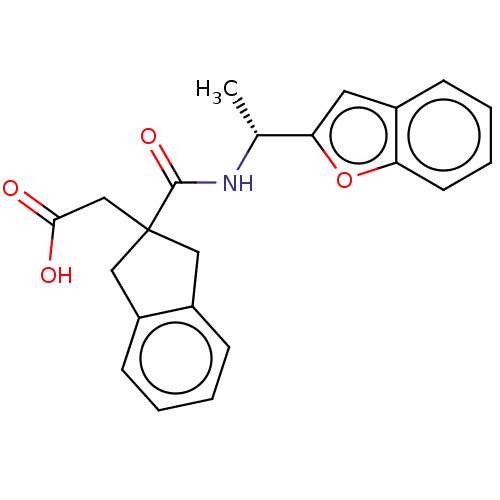
(CHEMBL4760989)Show SMILES C[C@@H](NC(=O)C1(CC(O)=O)Cc2ccccc2C1)c1cc2ccccc2o1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559568
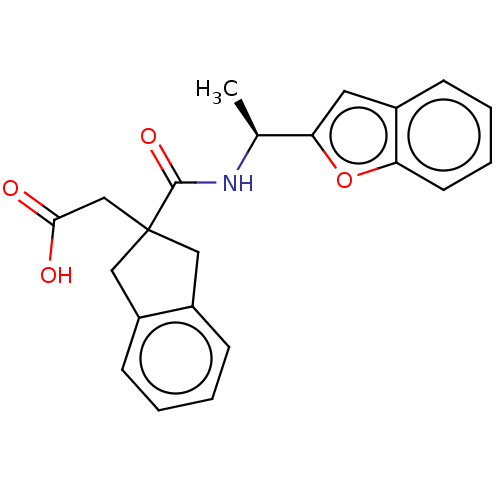
(CHEMBL4748733)Show SMILES C[C@H](NC(=O)C1(CC(O)=O)Cc2ccccc2C1)c1cc2ccccc2o1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559569

(CHEMBL4761199) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559570

(CHEMBL4760832) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559571

(CHEMBL4793389)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1ccc2ccccc2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559572

(CHEMBL4747047) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559573
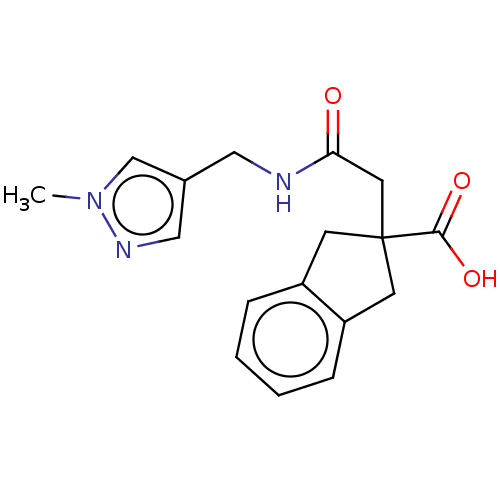
(CHEMBL4741361) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559574

(CHEMBL4785438)Show SMILES Cc1ccc2oc(CNC(=O)C3(CC(O)=O)Cc4ccccc4C3)cc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559575

(CHEMBL4798439)Show SMILES Cc1ccc2oc(CNC(=O)CC3(Cc4ccccc4C3)C(O)=O)cc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559576

(CHEMBL4794850)Show SMILES CC(NC(=O)CC1(Cc2ccccc2C1)C(O)=O)c1cc2ccccc2o1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559577

(CHEMBL4797456) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559554
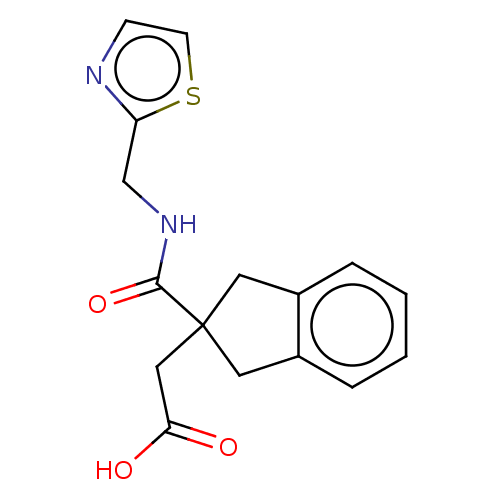
(CHEMBL4751454) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559578
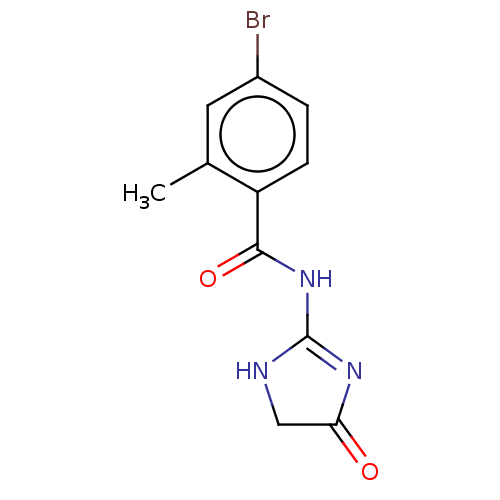
(CHEMBL4751618) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559551
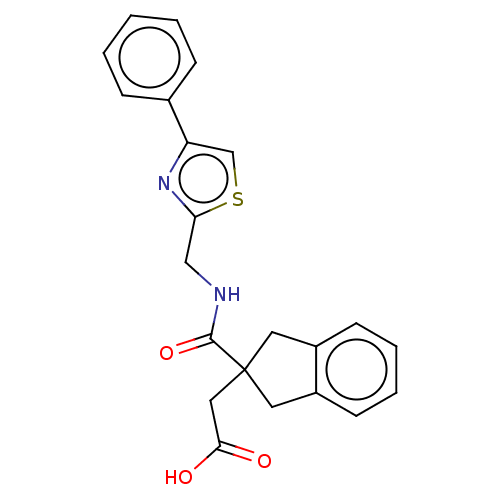
(CHEMBL4758612)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1nc(cs1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559553
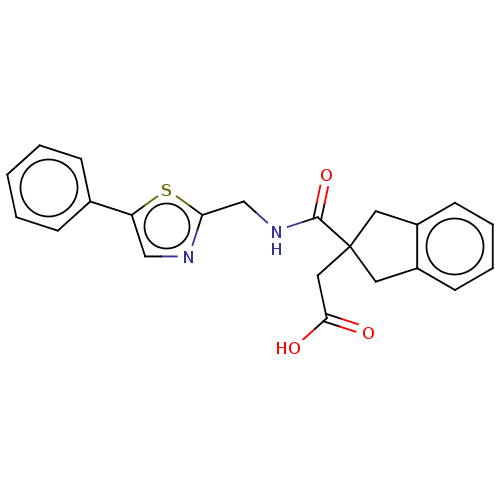
(CHEMBL4745918)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1ncc(s1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50539187

(CHEMBL4632769)Show SMILES Cc1cc(C)c(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1ncc[nH]1 Show InChI InChI=1S/C24H22N4O2/c1-16-13-17(2)22(14-21(16)23-26-11-12-27-23)28-24(29)18-6-8-20(9-7-18)30-15-19-5-3-4-10-25-19/h3-14H,15H2,1-2H3,(H,26,27)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115227
BindingDB Entry DOI: 10.7270/Q2CF9TNC |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50539188
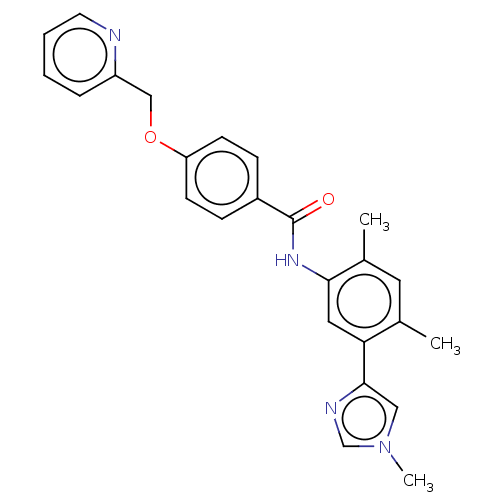
(CHEMBL4637222)Show SMILES Cc1cc(C)c(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1cn(C)cn1 Show InChI InChI=1S/C25H24N4O2/c1-17-12-18(2)23(13-22(17)24-14-29(3)16-27-24)28-25(30)19-7-9-21(10-8-19)31-15-20-6-4-5-11-26-20/h4-14,16H,15H2,1-3H3,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115227
BindingDB Entry DOI: 10.7270/Q2CF9TNC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50502619
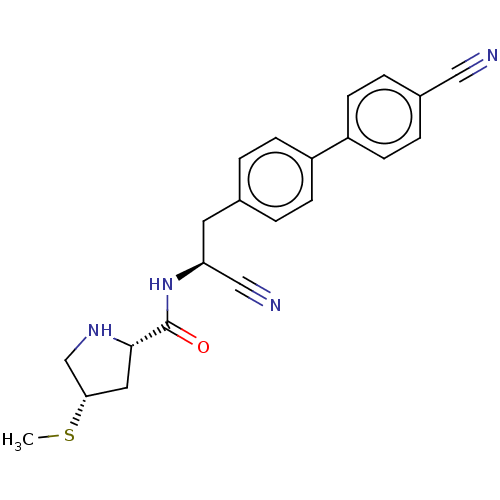
(CHEMBL4561264)Show SMILES CS[C@@H]1CN[C@@H](C1)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccc(cc1)C#N)C#N |r| Show InChI InChI=1S/C22H22N4OS/c1-28-20-11-21(25-14-20)22(27)26-19(13-24)10-15-2-6-17(7-3-15)18-8-4-16(12-23)5-9-18/h2-9,19-21,25H,10-11,14H2,1H3,(H,26,27)/t19-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPPI using H-Gly-Arg-AFC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins ... |
ACS Med Chem Lett 10: 1222-1227 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00261
BindingDB Entry DOI: 10.7270/Q2WM1HPF |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50249522

(2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(me...)Show SMILES CS(=O)(=O)c1ccc(C(=O)Nc2ccc(Cl)c(c2)-c2ccccn2)c(Cl)c1 Show InChI InChI=1S/C19H14Cl2N2O3S/c1-27(25,26)13-6-7-14(17(21)11-13)19(24)23-12-5-8-16(20)15(10-12)18-4-2-3-9-22-18/h2-11H,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115227
BindingDB Entry DOI: 10.7270/Q2CF9TNC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50502622
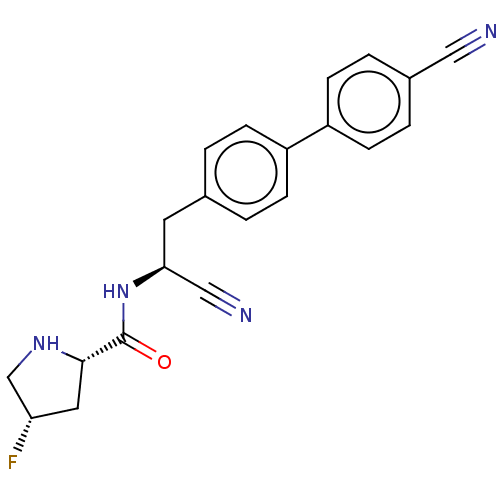
(CHEMBL4579467)Show SMILES F[C@@H]1CN[C@@H](C1)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccc(cc1)C#N)C#N |r| Show InChI InChI=1S/C21H19FN4O/c22-18-10-20(25-13-18)21(27)26-19(12-24)9-14-1-5-16(6-2-14)17-7-3-15(11-23)4-8-17/h1-8,18-20,25H,9-10,13H2,(H,26,27)/t18-,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPPI using H-Gly-Arg-AFC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins ... |
ACS Med Chem Lett 10: 1222-1227 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00261
BindingDB Entry DOI: 10.7270/Q2WM1HPF |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50388722
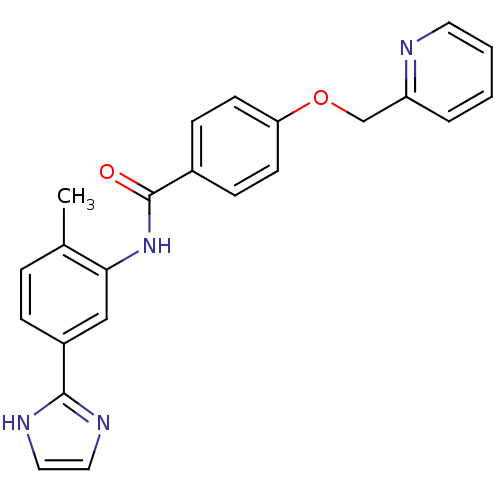
(CHEMBL2059865)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1ncc[nH]1 Show InChI InChI=1S/C23H20N4O2/c1-16-5-6-18(22-25-12-13-26-22)14-21(16)27-23(28)17-7-9-20(10-8-17)29-15-19-4-2-3-11-24-19/h2-14H,15H2,1H3,(H,25,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115227
BindingDB Entry DOI: 10.7270/Q2CF9TNC |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605537

(CHEMBL5183988)Show SMILES [H][C@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:4.4,1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;6.05,-1.47,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605543

(CHEMBL5207711)Show SMILES [H][C@]12CN(CCN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527057

(CHEMBL4461434)Show SMILES [H][C@]12CN([C@H](C)CN1c1c(cnc3c(F)c(c(Cl)cc13)-c1c(O)cccc1F)N(C)C2=O)C(=O)C=C |r,wU:1.0,4.4,(50.79,-26.84,;49.46,-27.62,;49.45,-26.08,;48.12,-25.32,;46.79,-26.1,;45.45,-25.34,;46.8,-27.64,;48.13,-28.4,;48.14,-29.94,;49.48,-30.7,;49.49,-32.25,;48.15,-33.03,;46.82,-32.26,;45.48,-33.04,;45.49,-34.58,;44.15,-32.27,;44.15,-30.72,;42.82,-29.95,;45.48,-29.95,;46.81,-30.71,;42.82,-33.04,;41.49,-32.26,;41.49,-30.72,;40.15,-33.03,;40.15,-34.57,;41.49,-35.34,;42.82,-34.57,;44.15,-35.34,;50.81,-29.92,;52.14,-30.69,;50.8,-28.38,;52.13,-27.61,;48.11,-23.78,;46.77,-23.02,;49.44,-23,;49.43,-21.46,)| Show InChI InChI=1S/C25H21ClF2N4O3/c1-4-19(34)31-11-17-25(35)30(3)16-9-29-23-13(24(16)32(17)10-12(31)2)8-14(26)20(22(23)28)21-15(27)6-5-7-18(21)33/h4-9,12,17,33H,1,10-11H2,2-3H3/t12-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50502621
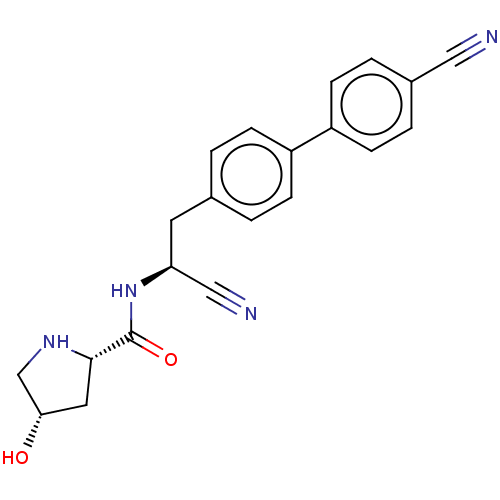
(CHEMBL4513306)Show SMILES O[C@@H]1CN[C@@H](C1)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccc(cc1)C#N)C#N |r| Show InChI InChI=1S/C21H20N4O2/c22-11-15-3-7-17(8-4-15)16-5-1-14(2-6-16)9-18(12-23)25-21(27)20-10-19(26)13-24-20/h1-8,18-20,24,26H,9-10,13H2,(H,25,27)/t18-,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPPI using H-Gly-Arg-AFC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins ... |
ACS Med Chem Lett 10: 1222-1227 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00261
BindingDB Entry DOI: 10.7270/Q2WM1HPF |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605540
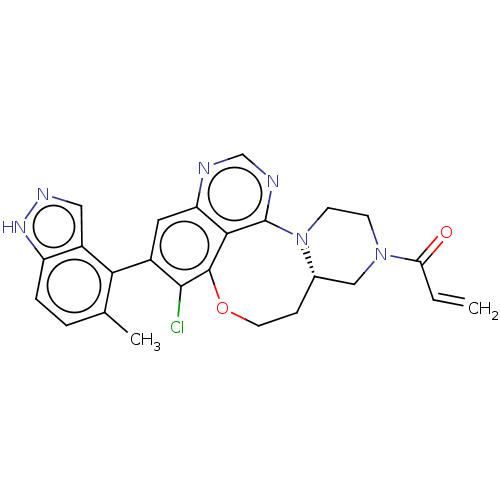
(CHEMBL5171553)Show SMILES [H][C@]12CN(CCN1c1ncnc3cc(c(Cl)c(OCC2)c13)-c1c(C)ccc2[nH]ncc12)C(=O)C=C |r,wD:1.0,(.27,4.4,;1.04,3.07,;1.88,4.35,;3.42,4.26,;4.11,2.89,;3.27,1.6,;1.73,1.69,;1.3,.21,;2.65,-.54,;2.67,-2.08,;1.35,-2.87,;0,-2.12,;-1.32,-2.91,;-2.66,-2.17,;-2.69,-.63,;-4.03,.12,;-1.37,.17,;-1.94,1.5,;-1.83,3.02,;-.41,3.6,;-.02,-.58,;-3.98,-2.96,;-5.33,-2.21,;-5.36,-.67,;-6.65,-3,;-6.62,-4.54,;-5.28,-5.29,;-4.93,-6.79,;-3.4,-6.93,;-2.8,-5.51,;-3.96,-4.5,;4.27,5.55,;3.58,6.93,;5.8,5.46,;6.65,6.75,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527051
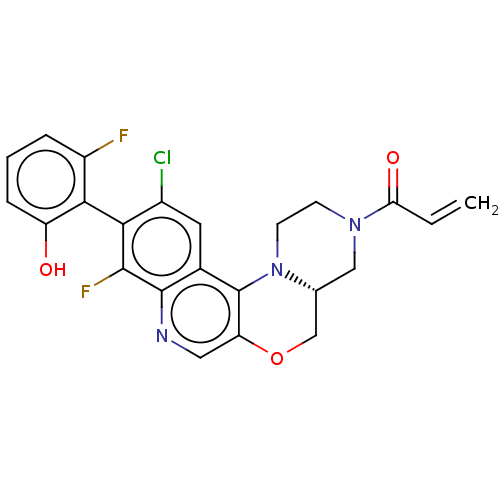
(CHEMBL4591572)Show SMILES [H][C@@]12COc3cnc4c(F)c(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,(13.3,-47.65,;11.97,-48.43,;13.31,-49.19,;13.32,-50.73,;11.99,-51.51,;12,-53.06,;10.66,-53.83,;9.33,-53.06,;7.99,-53.84,;8,-55.38,;6.66,-53.07,;6.66,-51.53,;5.33,-50.76,;7.99,-50.76,;9.33,-51.52,;10.65,-50.75,;10.64,-49.21,;9.31,-48.44,;9.3,-46.9,;10.63,-46.13,;11.97,-46.89,;10.62,-44.59,;9.28,-43.82,;11.95,-43.81,;11.94,-42.27,;5.33,-53.84,;4,-53.07,;4,-51.53,;2.66,-53.83,;2.66,-55.38,;4,-56.15,;5.33,-55.37,;6.67,-56.14,)| Show InChI InChI=1S/C23H18ClF2N3O3/c1-2-18(31)28-6-7-29-12(10-28)11-32-17-9-27-22-13(23(17)29)8-14(24)19(21(22)26)20-15(25)4-3-5-16(20)30/h2-5,8-9,12,30H,1,6-7,10-11H2/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527059
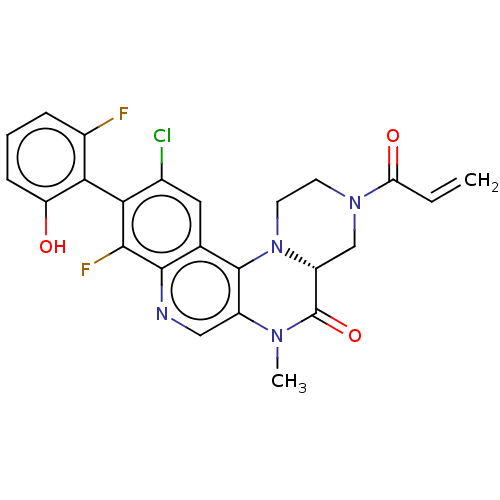
(CHEMBL4452974)Show SMILES [H][C@]12CN(CCN1c1c(cnc3c(F)c(c(Cl)cc13)-c1c(O)cccc1F)N(C)C2=O)C(=O)C=C |r,wU:1.0,(30.86,-26.9,;29.54,-27.68,;29.53,-26.14,;28.19,-25.38,;26.86,-26.15,;26.87,-27.69,;28.21,-28.46,;28.22,-30,;29.55,-30.76,;29.56,-32.31,;28.23,-33.08,;26.89,-32.31,;25.56,-33.09,;25.56,-34.63,;24.22,-32.32,;24.22,-30.78,;22.89,-30.01,;25.55,-30.01,;26.89,-30.77,;22.89,-33.09,;21.56,-32.32,;21.56,-30.78,;20.22,-33.08,;20.22,-34.62,;21.57,-35.4,;22.89,-34.62,;24.23,-35.39,;30.88,-29.98,;32.22,-30.74,;30.87,-28.44,;32.2,-27.66,;28.18,-23.84,;26.84,-23.07,;29.51,-23.06,;29.5,-21.52,)| Show InChI InChI=1S/C24H19ClF2N4O3/c1-3-18(33)30-7-8-31-16(11-30)24(34)29(2)15-10-28-22-12(23(15)31)9-13(25)19(21(22)27)20-14(26)5-4-6-17(20)32/h3-6,9-10,16,32H,1,7-8,11H2,2H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50521249
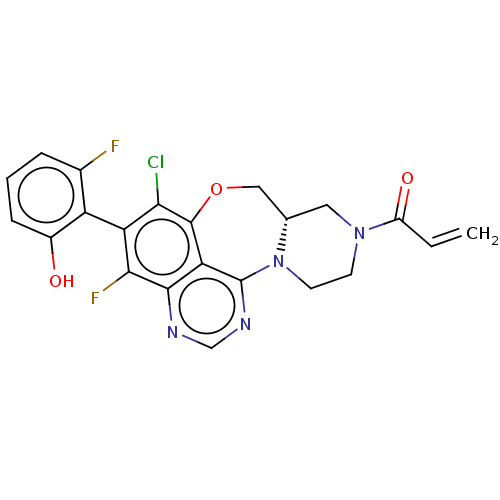
(CHEMBL4452819)Show SMILES [H][C@@]12CN(CCN1c1ncnc3c(F)c(c(Cl)c(OC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,(15.59,-28.29,;14.81,-29.62,;16.34,-29.49,;17.21,-30.76,;16.55,-32.15,;15.02,-32.27,;14.16,-31.01,;12.73,-31.25,;12.79,-32.74,;11.44,-33.52,;10.11,-32.74,;10.11,-31.19,;8.78,-30.44,;7.45,-31.21,;8.77,-28.91,;10.1,-28.13,;10.1,-26.58,;11.43,-28.89,;12.63,-27.92,;14.14,-28.24,;11.45,-30.43,;7.43,-28.14,;6.1,-28.93,;6.12,-30.46,;4.76,-28.17,;4.74,-26.61,;6.08,-25.83,;7.42,-26.6,;8.76,-25.82,;18.75,-30.64,;19.42,-29.24,;19.63,-31.91,;21.17,-31.78,)| Show InChI InChI=1S/C22H17ClF2N4O3/c1-2-14(31)28-6-7-29-11(8-28)9-32-21-17-20(26-10-27-22(17)29)19(25)16(18(21)23)15-12(24)4-3-5-13(15)30/h2-5,10-11,30H,1,6-9H2/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50605537

(CHEMBL5183988)Show SMILES [H][C@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:4.4,1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;6.05,-1.47,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50502616
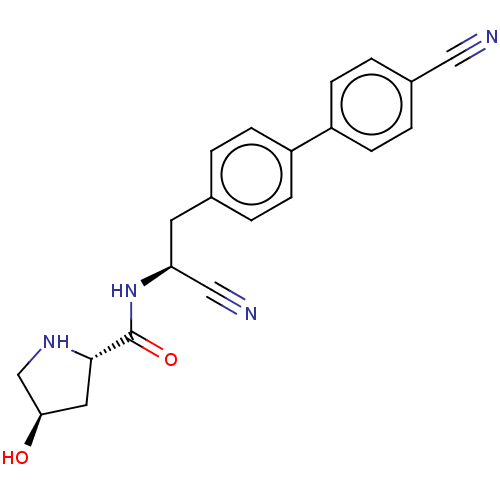
(CHEMBL4564319)Show SMILES O[C@H]1CN[C@@H](C1)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccc(cc1)C#N)C#N |r| Show InChI InChI=1S/C21H20N4O2/c22-11-15-3-7-17(8-4-15)16-5-1-14(2-6-16)9-18(12-23)25-21(27)20-10-19(26)13-24-20/h1-8,18-20,24,26H,9-10,13H2,(H,25,27)/t18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPPI using H-Gly-Arg-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins ... |
ACS Med Chem Lett 10: 1222-1227 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00261
BindingDB Entry DOI: 10.7270/Q2WM1HPF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50502617
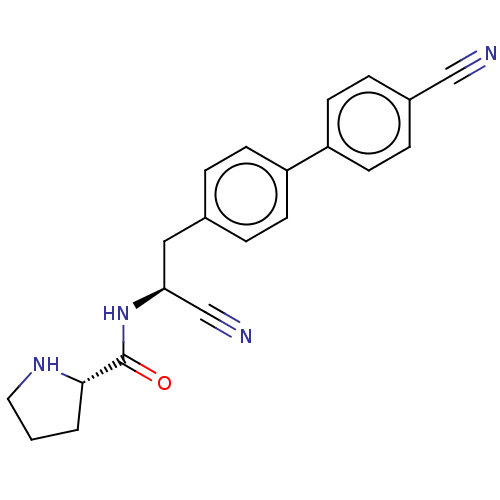
(CHEMBL4570759)Show SMILES O=C(N[C@@H](Cc1ccc(cc1)-c1ccc(cc1)C#N)C#N)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C21H20N4O/c22-13-16-5-9-18(10-6-16)17-7-3-15(4-8-17)12-19(14-23)25-21(26)20-2-1-11-24-20/h3-10,19-20,24H,1-2,11-12H2,(H,25,26)/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPPI using H-Gly-Arg-AFC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins ... |
ACS Med Chem Lett 10: 1222-1227 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00261
BindingDB Entry DOI: 10.7270/Q2WM1HPF |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527058
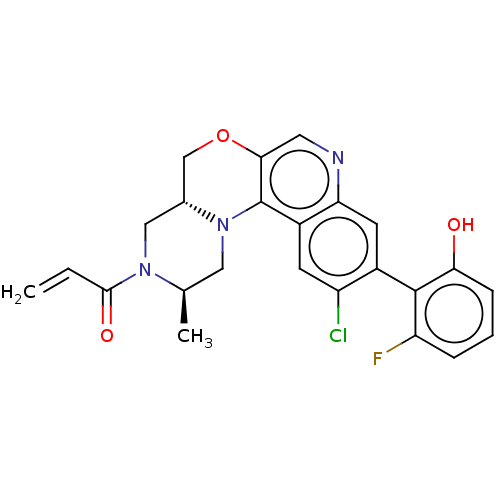
(CHEMBL4455191)Show SMILES [H][C@@]12COc3cnc4cc(c(Cl)cc4c3N1C[C@@H](C)N(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,17.20,(70.37,-6.5,;69.05,-7.29,;70.38,-8.05,;70.39,-9.59,;69.06,-10.36,;69.07,-11.91,;67.74,-12.69,;66.4,-11.92,;65.07,-12.7,;63.73,-11.93,;63.73,-10.38,;62.4,-9.61,;65.06,-9.61,;66.4,-10.38,;67.72,-9.6,;67.72,-8.06,;66.38,-7.3,;66.37,-5.76,;65.03,-5,;67.7,-4.98,;69.04,-5.75,;67.69,-3.44,;66.35,-2.68,;69.02,-2.67,;69.01,-1.13,;62.4,-12.7,;61.07,-11.92,;61.07,-10.38,;59.73,-12.69,;59.73,-14.23,;61.08,-15,;62.4,-14.23,;63.74,-15,)| Show InChI InChI=1S/C24H21ClFN3O3/c1-3-22(31)28-11-14-12-32-21-9-27-19-8-15(23-18(26)5-4-6-20(23)30)17(25)7-16(19)24(21)29(14)10-13(28)2/h3-9,13-14,30H,1,10-12H2,2H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527050
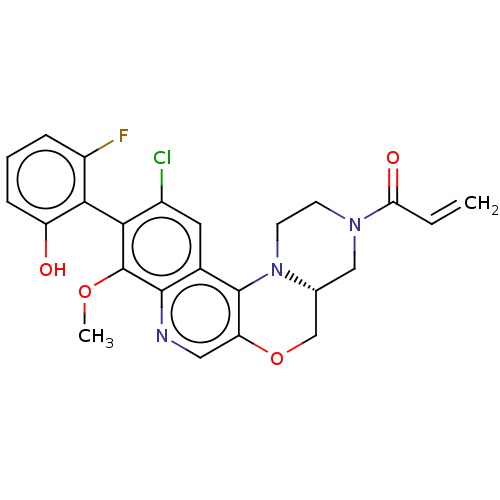
(CHEMBL4572730)Show SMILES [H][C@@]12COc3cnc4c(OC)c(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,(51.05,-48.22,;49.73,-49.01,;51.06,-49.76,;51.07,-51.3,;49.74,-52.08,;49.75,-53.63,;48.42,-54.41,;47.08,-53.64,;45.75,-54.42,;45.75,-55.96,;47.09,-56.72,;44.41,-53.65,;44.42,-52.1,;43.08,-51.33,;45.74,-51.33,;47.08,-52.1,;48.41,-51.32,;48.4,-49.78,;47.06,-49.02,;47.05,-47.48,;48.38,-46.7,;49.72,-47.47,;48.37,-45.16,;47.04,-44.4,;49.7,-44.39,;49.69,-42.85,;43.08,-54.42,;41.75,-53.64,;41.75,-52.1,;40.42,-54.41,;40.41,-55.95,;41.76,-56.72,;43.08,-55.95,;44.42,-56.72,)| Show InChI InChI=1S/C24H21ClFN3O4/c1-3-19(31)28-7-8-29-13(11-28)12-33-18-10-27-22-14(23(18)29)9-15(25)20(24(22)32-2)21-16(26)5-4-6-17(21)30/h3-6,9-10,13,30H,1,7-8,11-12H2,2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527054
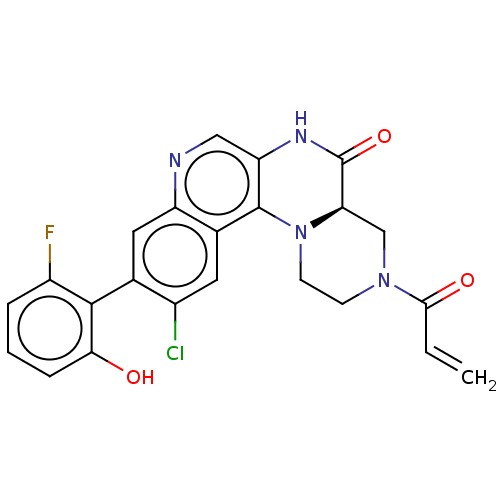
(CHEMBL4453777)Show SMILES [H][C@]12CN(CCN1c1c(NC2=O)cnc2cc(c(Cl)cc12)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,(31.71,-6.52,;30.38,-7.3,;30.38,-5.76,;29.04,-5,;27.71,-5.78,;27.72,-7.32,;29.05,-8.08,;29.06,-9.62,;30.4,-10.38,;31.73,-9.6,;31.72,-8.06,;33.05,-7.28,;30.41,-11.93,;29.07,-12.7,;27.74,-11.94,;26.4,-12.71,;25.07,-11.94,;25.07,-10.4,;23.74,-9.63,;26.4,-9.63,;27.74,-10.39,;23.74,-12.71,;22.41,-11.94,;22.41,-10.4,;21.07,-12.71,;21.07,-14.25,;22.41,-15.02,;23.74,-14.25,;25.08,-15.01,;29.03,-3.46,;27.69,-2.7,;30.36,-2.68,;30.35,-1.14,)| Show InChI InChI=1S/C23H18ClFN4O3/c1-2-20(31)28-6-7-29-18(11-28)23(32)27-17-10-26-16-9-12(14(24)8-13(16)22(17)29)21-15(25)4-3-5-19(21)30/h2-5,8-10,18,30H,1,6-7,11H2,(H,27,32)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527054

(CHEMBL4453777)Show SMILES [H][C@]12CN(CCN1c1c(NC2=O)cnc2cc(c(Cl)cc12)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,(31.71,-6.52,;30.38,-7.3,;30.38,-5.76,;29.04,-5,;27.71,-5.78,;27.72,-7.32,;29.05,-8.08,;29.06,-9.62,;30.4,-10.38,;31.73,-9.6,;31.72,-8.06,;33.05,-7.28,;30.41,-11.93,;29.07,-12.7,;27.74,-11.94,;26.4,-12.71,;25.07,-11.94,;25.07,-10.4,;23.74,-9.63,;26.4,-9.63,;27.74,-10.39,;23.74,-12.71,;22.41,-11.94,;22.41,-10.4,;21.07,-12.71,;21.07,-14.25,;22.41,-15.02,;23.74,-14.25,;25.08,-15.01,;29.03,-3.46,;27.69,-2.7,;30.36,-2.68,;30.35,-1.14,)| Show InChI InChI=1S/C23H18ClFN4O3/c1-2-20(31)28-6-7-29-18(11-28)23(32)27-17-10-26-16-9-12(14(24)8-13(16)22(17)29)21-15(25)4-3-5-19(21)30/h2-5,8-10,18,30H,1,6-7,11H2,(H,27,32)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KRAS G12C mutant (unknown origin) |
Citation and Details
Article DOI: 10.1039/d1md00055a
BindingDB Entry DOI: 10.7270/Q2DB85KH |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50502620
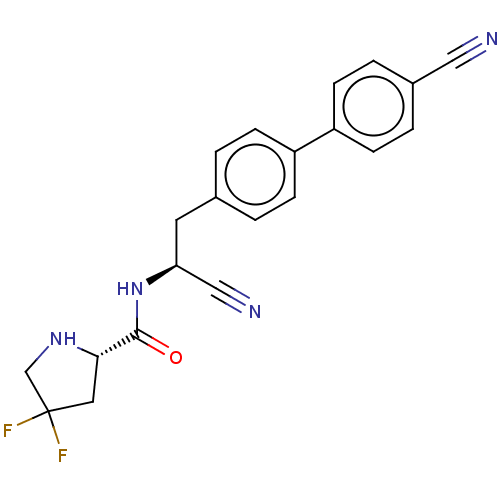
(CHEMBL4451898)Show SMILES FC1(F)CN[C@@H](C1)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccc(cc1)C#N)C#N |r| Show InChI InChI=1S/C21H18F2N4O/c22-21(23)10-19(26-13-21)20(28)27-18(12-25)9-14-1-5-16(6-2-14)17-7-3-15(11-24)4-8-17/h1-8,18-19,26H,9-10,13H2,(H,27,28)/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPPI using H-Gly-Arg-AFC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins ... |
ACS Med Chem Lett 10: 1222-1227 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00261
BindingDB Entry DOI: 10.7270/Q2WM1HPF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data