

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human DHFR using 45 uM DHF as substrate by spectrophotometry | ACS Med Chem Lett 6: 1140-4 (2015) Article DOI: 10.1021/acsmedchemlett.5b00367 BindingDB Entry DOI: 10.7270/Q25H7J3Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mycobacterium tuberculosis) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Mycobacterium tuberculosis DHFR using 45 uM DHF as substrate by spectrophotometry | ACS Med Chem Lett 6: 1140-4 (2015) Article DOI: 10.1021/acsmedchemlett.5b00367 BindingDB Entry DOI: 10.7270/Q25H7J3Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233037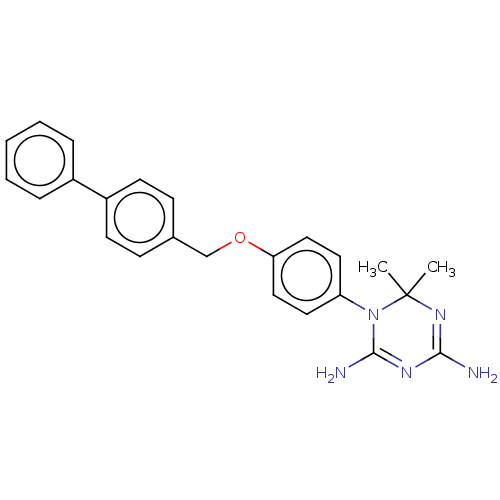 (1-(4-(Biphenyl-4-ylmethoxy)phenyl)-6,6-dimethyl-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233034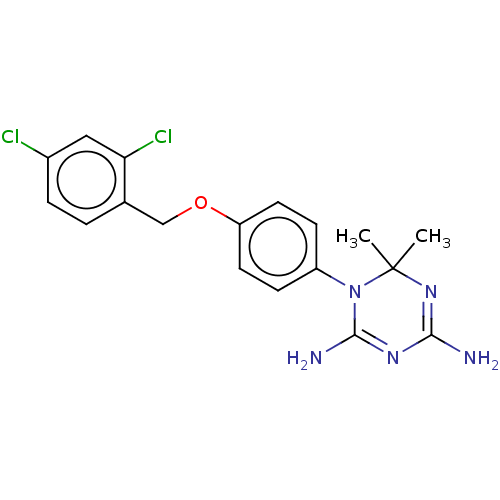 (1-(4-(2,4-Dichlorobenzyloxy)phenyl)-6,6-dimethyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233036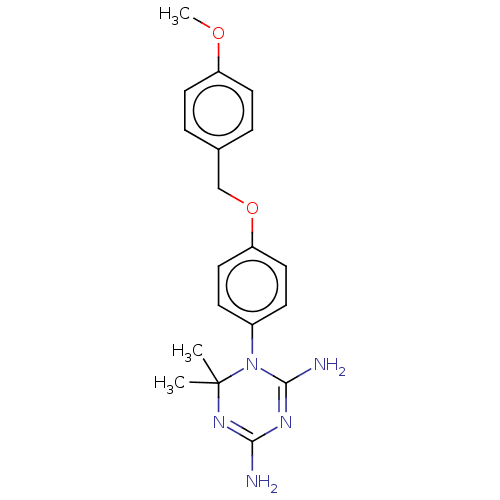 (1-(4-(4-Methoxybenzyloxy)phenyl)-6,6-dimethyl-1,6-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233032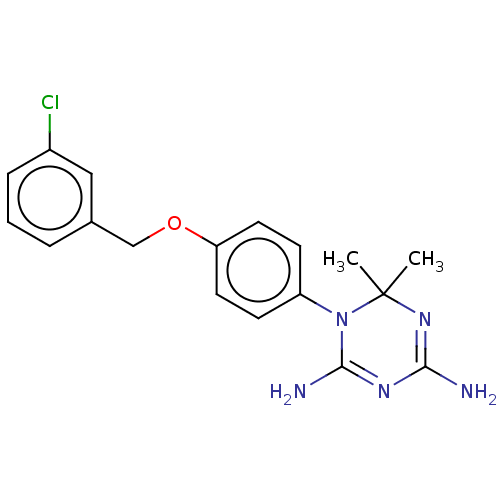 (1-(4-(3-Chlorobenzyloxy)phenyl)-6,6-dimethyl-1,6-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233037 (1-(4-(Biphenyl-4-ylmethoxy)phenyl)-6,6-dimethyl-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233034 (1-(4-(2,4-Dichlorobenzyloxy)phenyl)-6,6-dimethyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50405043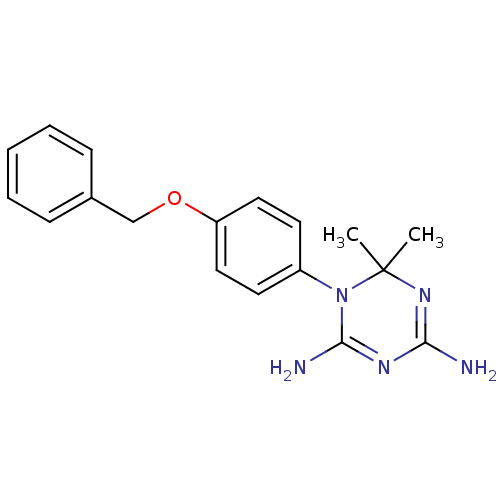 (1-(4-(Benzyloxy)phenyl)-6,6-dimethyl-1,6-dihydro-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233035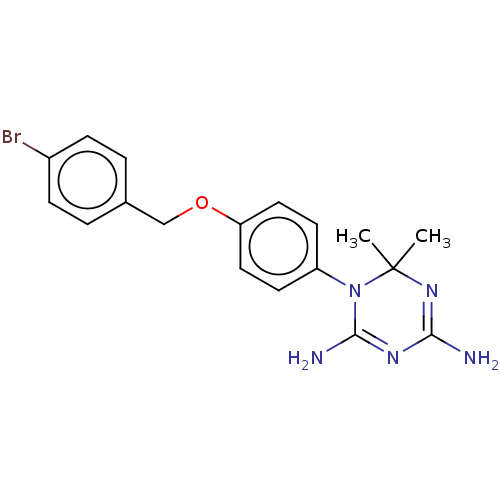 (1-(4-(4-Bromobenzyloxy)phenyl)-6,6-dimethyl-1,6-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233023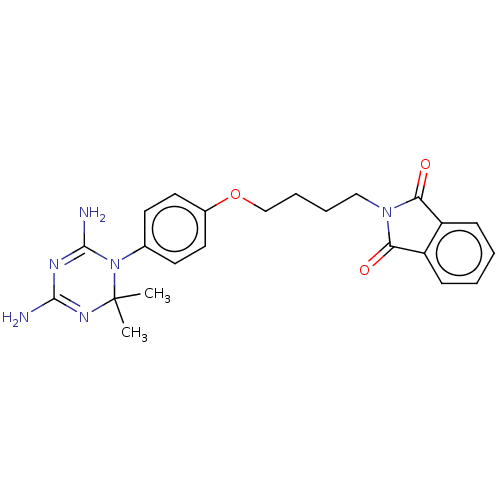 (2-(4-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233035 (1-(4-(4-Bromobenzyloxy)phenyl)-6,6-dimethyl-1,6-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233032 (1-(4-(3-Chlorobenzyloxy)phenyl)-6,6-dimethyl-1,6-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233033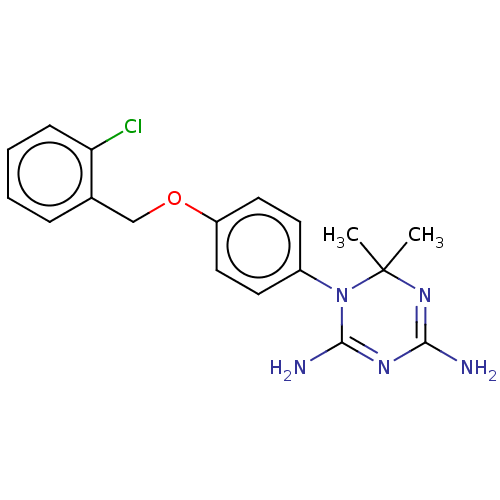 (1-(4-(2-Chlorobenzyloxy)phenyl)-6,6-dimethyl-1,6-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233036 (1-(4-(4-Methoxybenzyloxy)phenyl)-6,6-dimethyl-1,6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233033 (1-(4-(2-Chlorobenzyloxy)phenyl)-6,6-dimethyl-1,6-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233023 (2-(4-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50405043 (1-(4-(Benzyloxy)phenyl)-6,6-dimethyl-1,6-dihydro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233022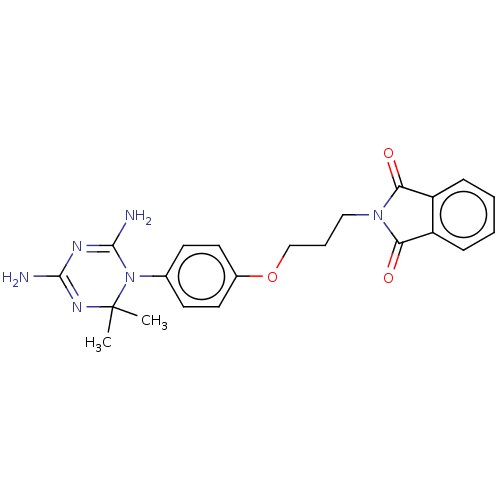 (2-(3-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HTH-type transcriptional regulator EthR (Mycobacterium tuberculosis) | BDBM50363052 (CHEMBL1946956) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv EthR | Bioorg Med Chem Lett 29: 1999-2007 (2019) Article DOI: 10.1016/j.bmcl.2019.06.054 BindingDB Entry DOI: 10.7270/Q27S7S9X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233037 (1-(4-(Biphenyl-4-ylmethoxy)phenyl)-6,6-dimethyl-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233021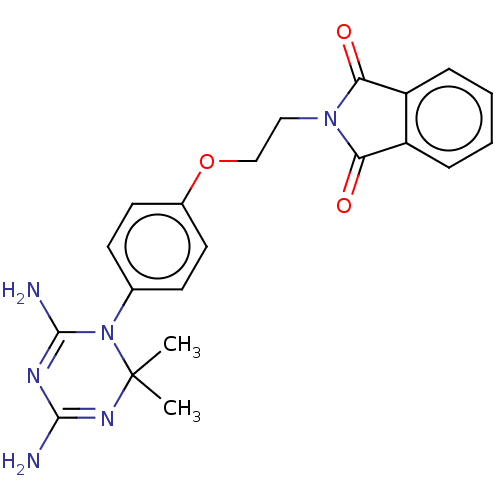 (2-(2-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233024 (2-(3-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 523 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233022 (2-(3-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 556 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233035 (1-(4-(4-Bromobenzyloxy)phenyl)-6,6-dimethyl-1,6-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 829 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233034 (1-(4-(2,4-Dichlorobenzyloxy)phenyl)-6,6-dimethyl-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233032 (1-(4-(3-Chlorobenzyloxy)phenyl)-6,6-dimethyl-1,6-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233021 (2-(2-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233036 (1-(4-(4-Methoxybenzyloxy)phenyl)-6,6-dimethyl-1,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233024 (2-(3-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233033 (1-(4-(2-Chlorobenzyloxy)phenyl)-6,6-dimethyl-1,6-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233023 (2-(4-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM233036 (1-(4-(4-Methoxybenzyloxy)phenyl)-6,6-dimethyl-1,6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM233032 (1-(4-(3-Chlorobenzyloxy)phenyl)-6,6-dimethyl-1,6-d...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM50405043 (1-(4-(Benzyloxy)phenyl)-6,6-dimethyl-1,6-dihydro-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233022 (2-(3-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM233035 (1-(4-(4-Bromobenzyloxy)phenyl)-6,6-dimethyl-1,6-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233030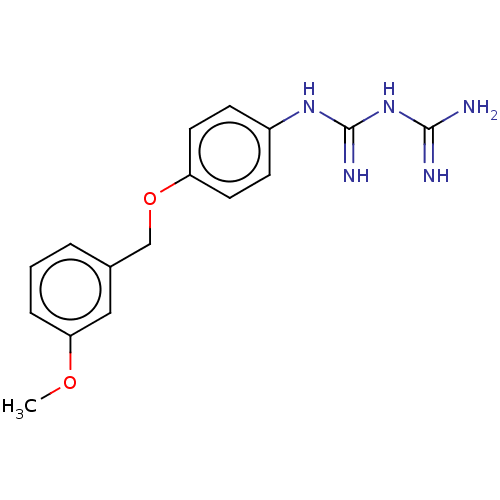 (N-{4-[(3-Methoxybenzyl)oxy]phenyl}imidodicarbonimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM233023 (2-(4-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233027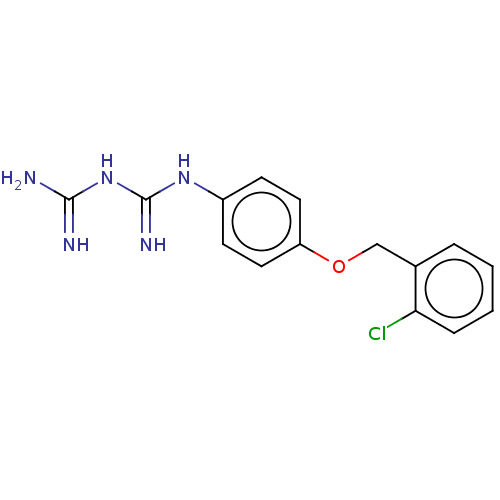 (N-{4-[(2-Chlorobenzyl)oxy]phenyl}imidodicarbonimid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM233037 (1-(4-(Biphenyl-4-ylmethoxy)phenyl)-6,6-dimethyl-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM233033 (1-(4-(2-Chlorobenzyloxy)phenyl)-6,6-dimethyl-1,6-d...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM233034 (1-(4-(2,4-Dichlorobenzyloxy)phenyl)-6,6-dimethyl-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM50405043 (1-(4-(Benzyloxy)phenyl)-6,6-dimethyl-1,6-dihydro-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233030 (N-{4-[(3-Methoxybenzyl)oxy]phenyl}imidodicarbonimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233021 (2-(2-(4-(4,6-Diamino-2,2-dimethyl-1,3,5-triazin-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM233030 (N-{4-[(3-Methoxybenzyl)oxy]phenyl}imidodicarbonimi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM233030 (N-{4-[(3-Methoxybenzyl)oxy]phenyl}imidodicarbonimi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM233027 (N-{4-[(2-Chlorobenzyl)oxy]phenyl}imidodicarbonimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM233029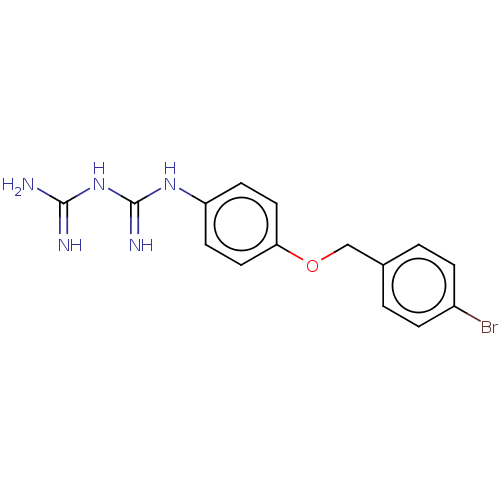 (N-{4-[(4-Bromobenzyl)oxy]phenyl}imidodicarbonimidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology | Assay Description The synthesized compounds were evaluated for their ability to inhibit DHFR from pc, tg, ma, and rl using a continuous spectrophotometric assay measur... | J Enzyme Inhib Med Chem 25: 331-9 (2010) Article DOI: 10.3109/14756360903179443 BindingDB Entry DOI: 10.7270/Q2QJ7G6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |