

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110170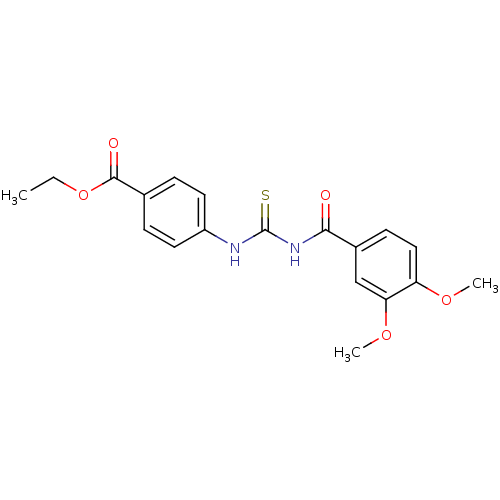 (Ethyl 4-[3-(3,4-dimethoxybenzoyl)thioureido]benzoa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50561931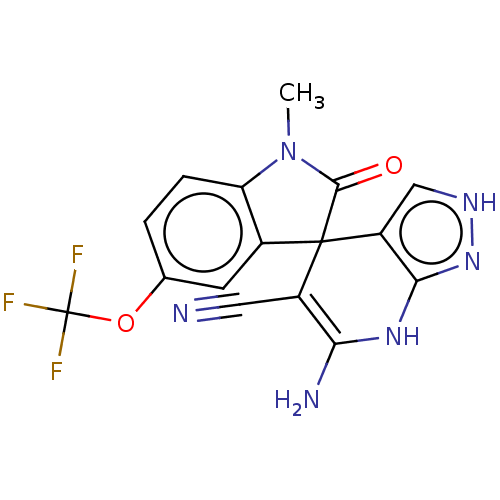 (CHEMBL4746318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50561933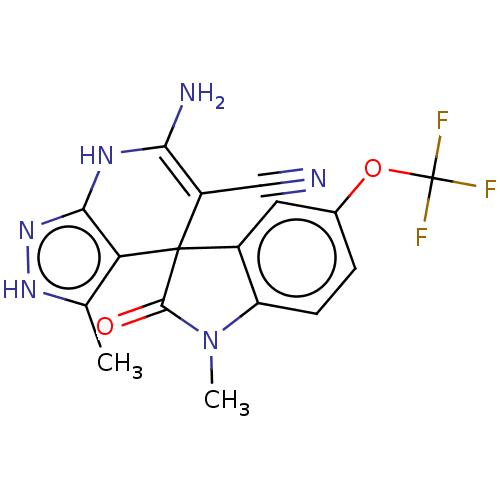 (CHEMBL4788305) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561936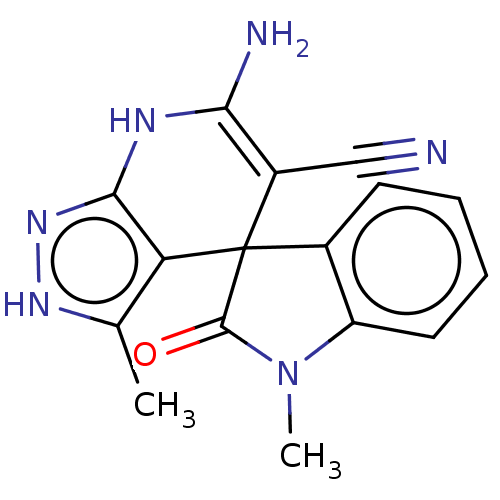 (CHEMBL4741833) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110169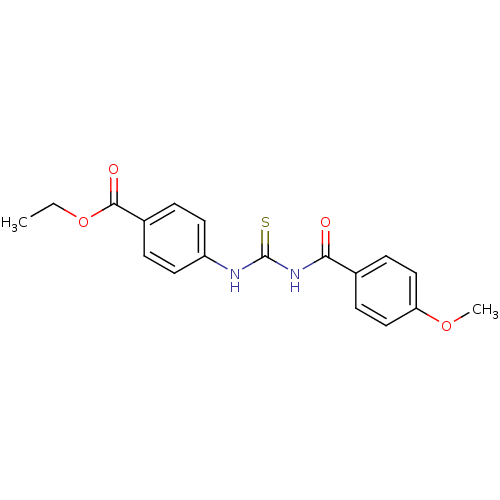 (Ethyl 4-[3-(4-methoxybenzoyl)thioureido]benzoate (...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110165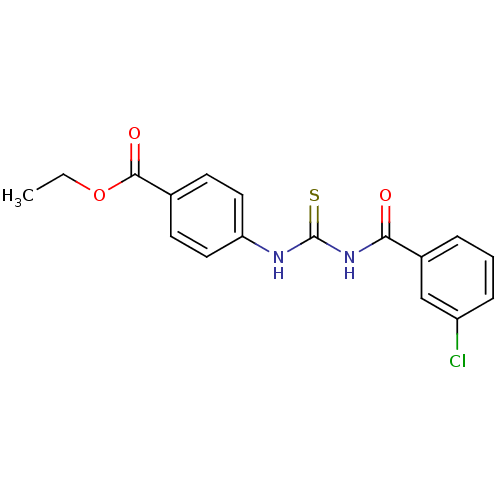 (Ethyl 4-[3-(3-chlorobenzoyl)thioureido]benzoate (1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50561932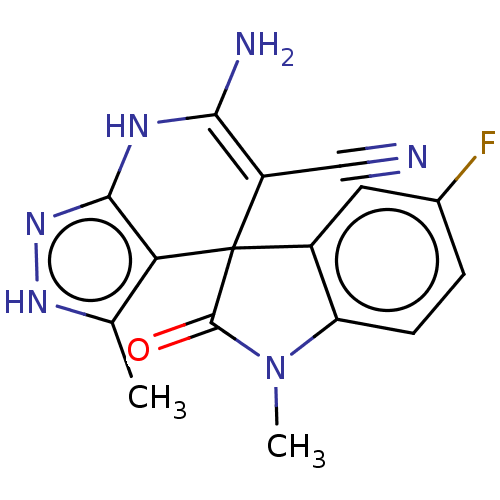 (CHEMBL4761451) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110166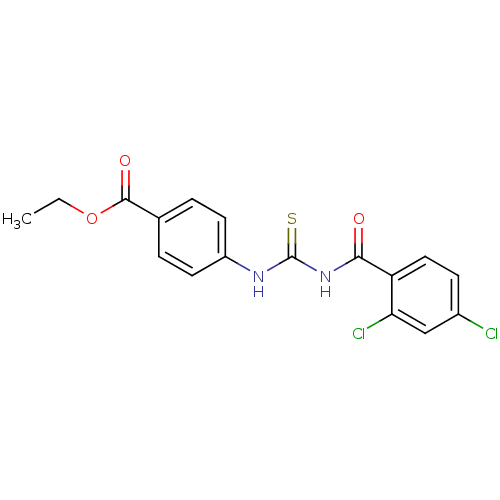 (Ethyl 4-[3-(2,4-dichlorobenzoyl)thioureido]benzoat...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003958 (CHEMBL3236084) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50561938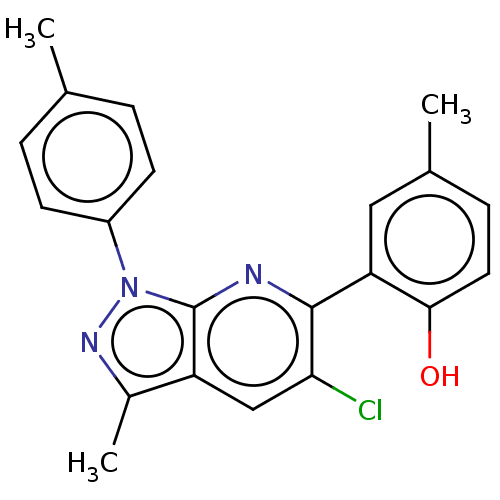 (CHEMBL4746633) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ecto-5' nucleotidase expressed in COS7 cell membranes using AMP as substrate preincubated for 10 mins followed by sub... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110171 (Ethyl 4-[3-(2-bromobenzoyl)thioureido]benzoate (1h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50561930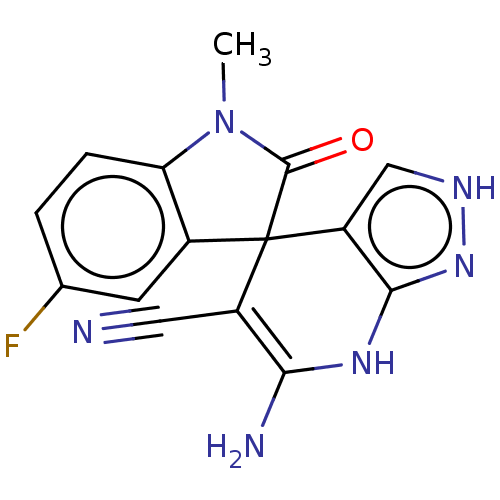 (CHEMBL4760778) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110172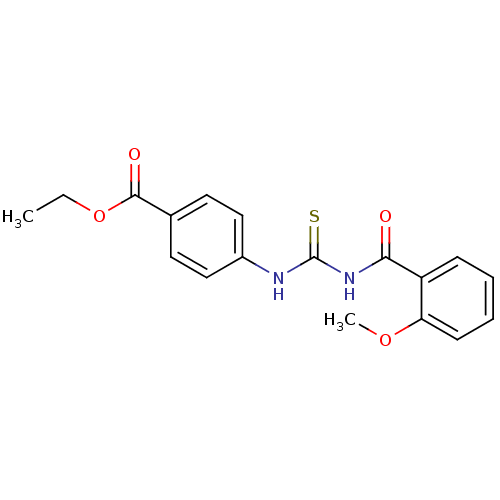 (Ethyl 4-[3-(3-methoxybenzoyl)thioureido]benzoate (...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110164 (Ethyl 4-(3-benzoylthioureido) benzoate (1a)) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561931 (CHEMBL4746318) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110167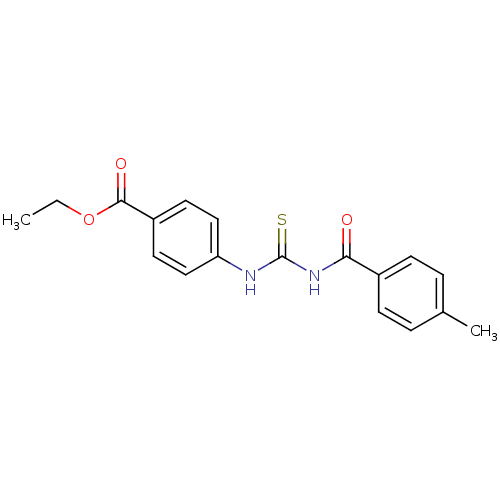 (Ethyl 4-[3-(4-methylbenzoyl)thioureido]benzoate (1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561934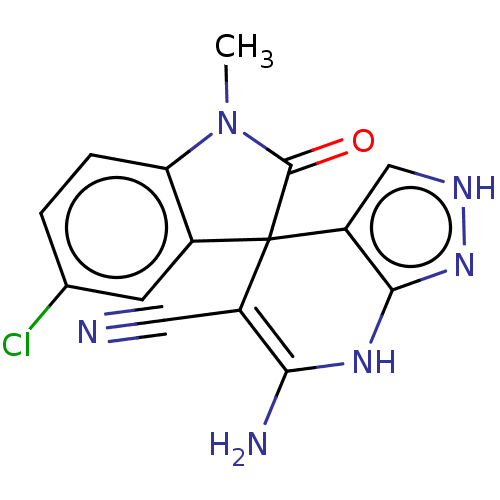 (CHEMBL4753669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50561929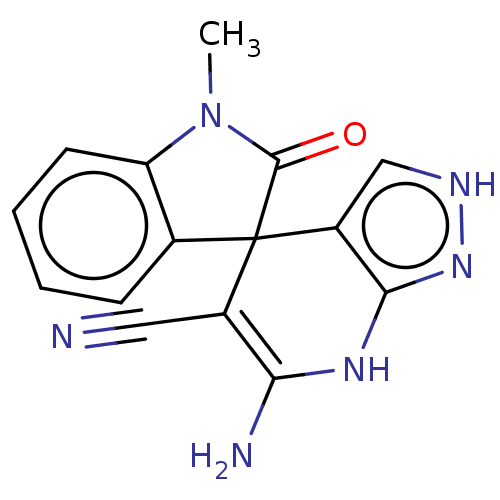 (CHEMBL4797703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110173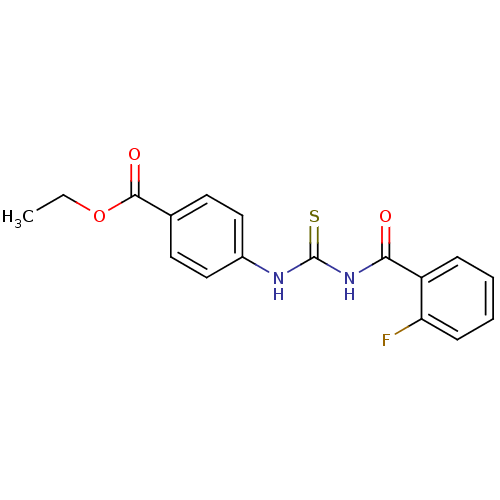 (Ethyl 4-[3-(2-fluorobenzoyl)thioureido]benzoate (1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110168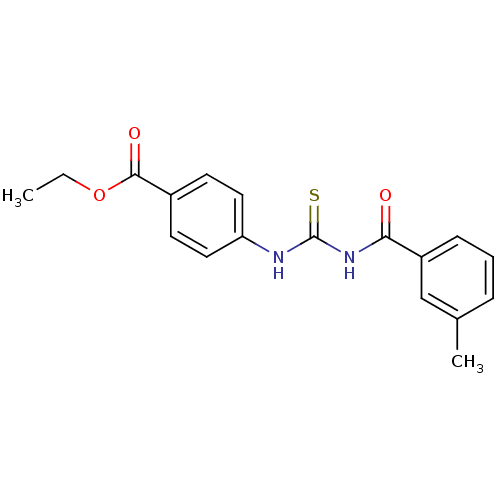 (Ethyl 4-[3-(3-methylbenzoyl)thioureido]benzoate (1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50003950 (CHEMBL3236082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50003953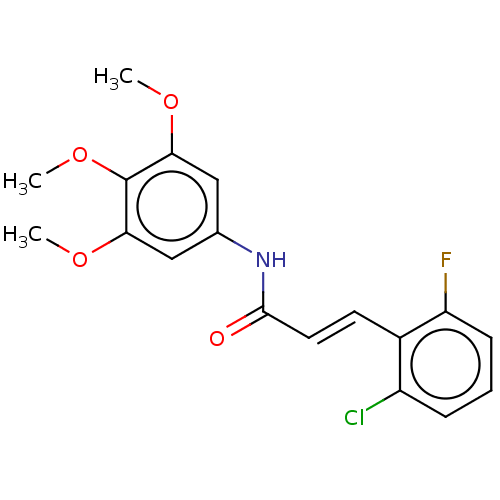 (CHEMBL3236097) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003960 (CHEMBL3236088) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561937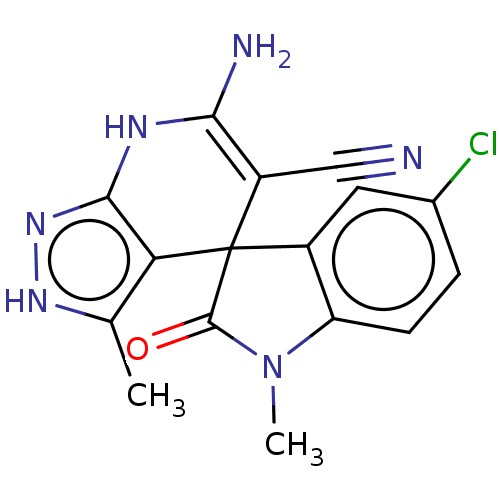 (CHEMBL4780721) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003957 (CHEMBL3236083) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561935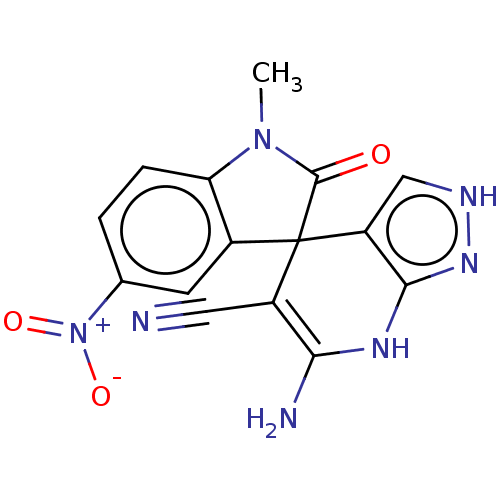 (CHEMBL4761355) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP/microtubule affinity-regulating kinase 4 (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00053a BindingDB Entry DOI: 10.7270/Q2222ZR4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003961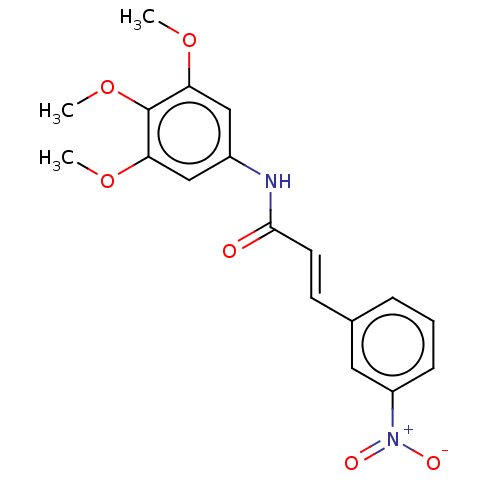 (CHEMBL3236090) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561933 (CHEMBL4788305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003964 (CHEMBL3236098) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50003949 (CHEMBL3235783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50003948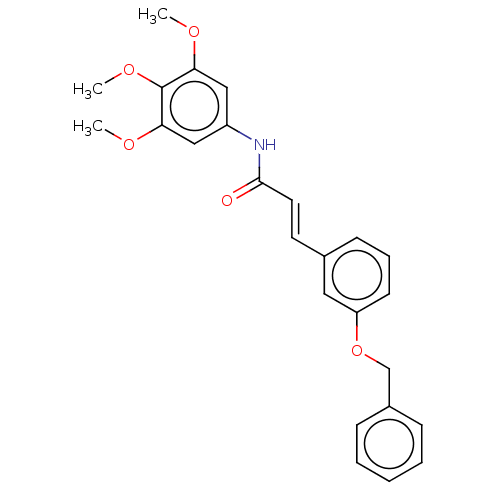 (CHEMBL1094079) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003952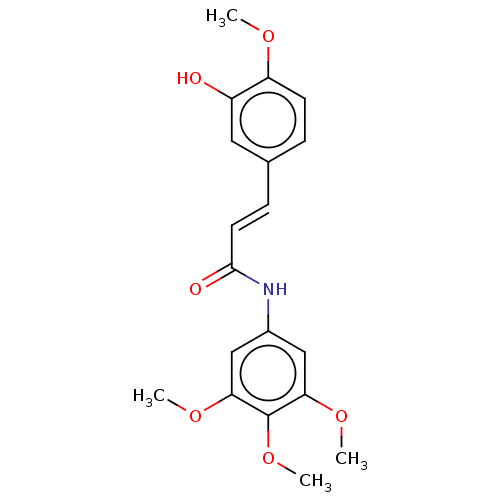 (CHEMBL3236093) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003954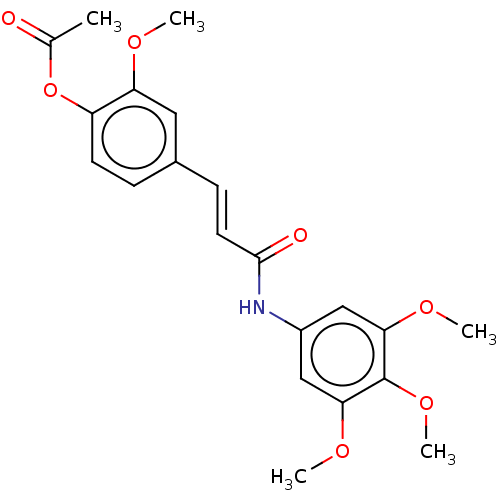 (CHEMBL3235782) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561932 (CHEMBL4761451) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50003951 (CHEMBL3236092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003956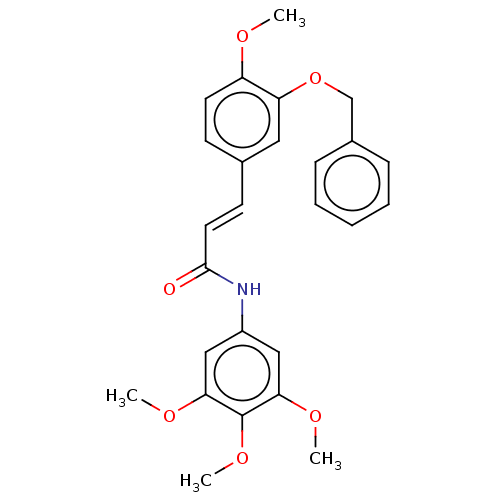 (CHEMBL3235785) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003951 (CHEMBL3236092) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003948 (CHEMBL1094079) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003965 (CHEMBL3236099) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50003952 (CHEMBL3236093) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561930 (CHEMBL4760778) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003949 (CHEMBL3235783) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003955 (CHEMBL3235784) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003959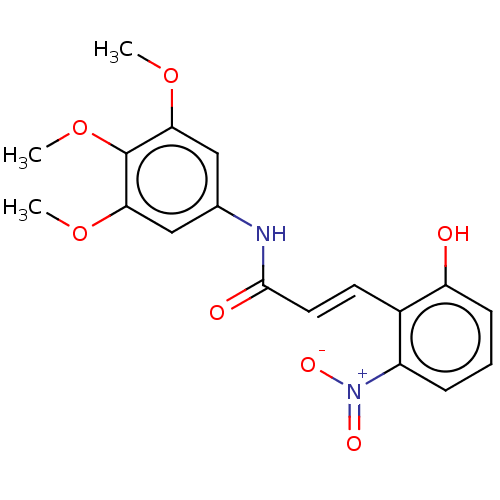 (CHEMBL3236087) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50003963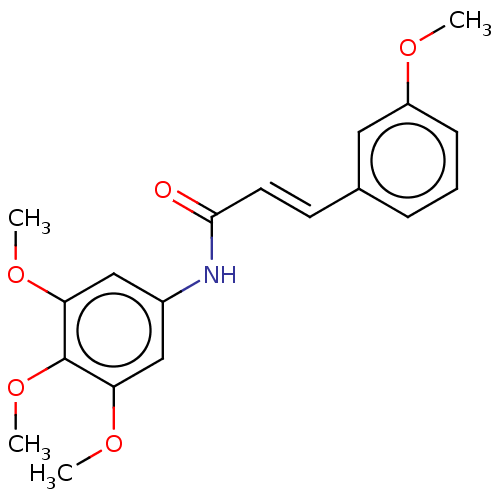 (CHEMBL1094988) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 43-53 (2014) Article DOI: 10.1016/j.ejmech.2014.03.015 BindingDB Entry DOI: 10.7270/Q2KW5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445973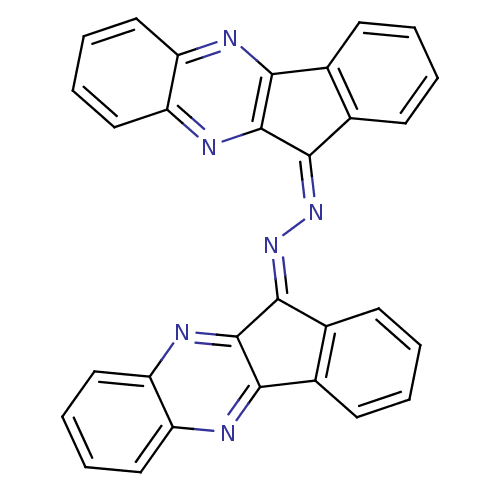 (CHEMBL3103095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease [D459Y,K653P] (Canavalia ensiformis (Jack bean)) | BDBM110174 ((2-chlorophenyl)thiourea | Thiourea) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | 8.2 | 37 |
Quaid-I-Azam University, Islamabad, Pakistan. | Assay Description The assay mixture contained 40 µL of buffer (0.01 M LiCl2, 1 mM EDTA, 0.01M K2HPO4, pH 8.2) containing 100 mM urea, 10 µL of enzyme (5U/mL)... | Bioorg Chem 52: 1-7 (2014) Article DOI: 10.1016/j.bioorg.2013.10.001 BindingDB Entry DOI: 10.7270/Q2X34W43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |