

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOA using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 15 mins by ... | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50199522 ((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756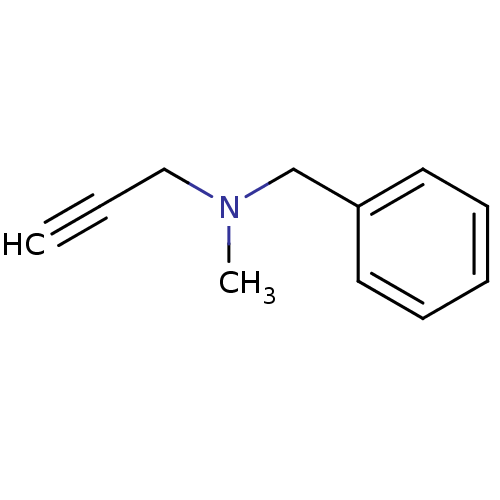 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate preincubated for 15 mins followed by substrate addition measured after 15 mins by... | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284434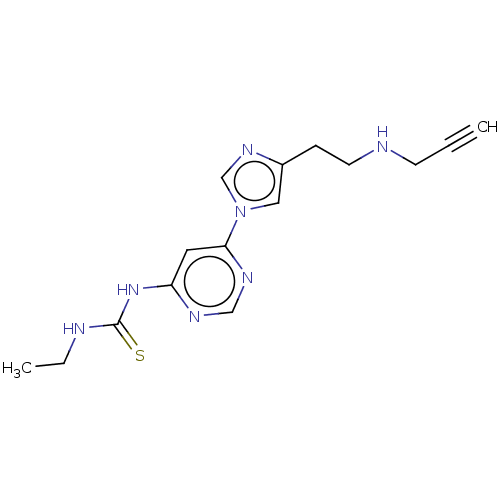 (CHEMBL4163995) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50082665 (4-(dimethylamino)-N-(7-(hydroxyamino)-7-oxoheptyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H aminopeptidase activity expressed in Escherichia coli BL21 (DE3) pLysS assessed as formation of p-NA from Ala-p... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284335 (CHEMBL4162742) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284433 (CHEMBL4174105) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 354 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284432 (CHEMBL4175437) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 381 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284336 (CHEMBL4174653) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284331 (CHEMBL4164807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284339 (CHEMBL4170678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284338 (CHEMBL4160334) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284430 (CHEMBL4174258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284334 (CHEMBL4170832) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 515 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284333 (CHEMBL4164119) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 585 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284424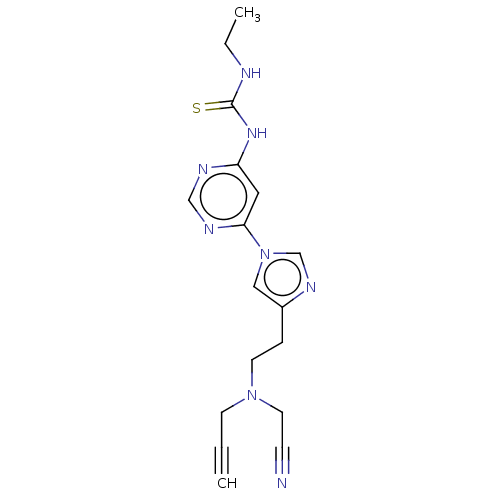 (CHEMBL4173774) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 597 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284329 (CHEMBL4166352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 675 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50082665 (4-(dimethylamino)-N-(7-(hydroxyamino)-7-oxoheptyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284332 (CHEMBL4167443) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 707 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284431 (CHEMBL4159540) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 718 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Mus musculus) | BDBM50082665 (4-(dimethylamino)-N-(7-(hydroxyamino)-7-oxoheptyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of LTA4H in C57BL/6 mouse assessed as reduction in LTB4 production pre-incubated for 30 mins before 5-(methylamino)-2-({(2R,3R,6S,8S,9R,11... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284337 (CHEMBL4169378) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenate using acetylthiocholine iodide as substrate after 20 mins by Ellman's method | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50284335 (CHEMBL4162742) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate preincubated for 15 mins followed by substrate addition measured after 15 mins by... | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H aminopeptidase activity expressed in Escherichia coli BL21 (DE3) pLysS assessed as formation of p-NA from Ala-p... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50284331 (CHEMBL4164807) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate preincubated for 15 mins followed by substrate addition measured after 15 mins by... | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50284433 (CHEMBL4174105) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOA using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 15 mins by ... | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50328678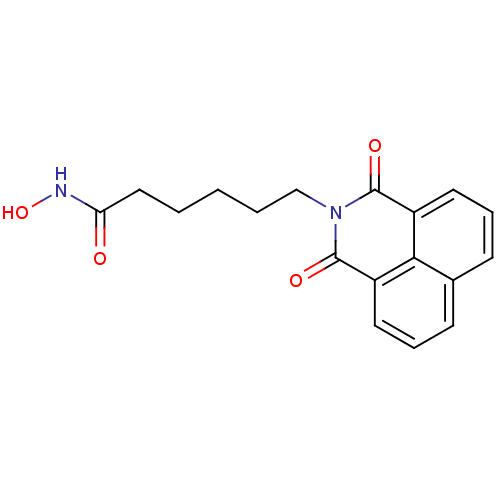 (6-(1,3-Dioxo-1H,3H-benzo[de]isoquinolin-2-yl)-hexa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H aminopeptidase activity expressed in Escherichia coli BL21 (DE3) pLysS assessed as formation of p-NA from Ala-p... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50284433 (CHEMBL4174105) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate preincubated for 15 mins followed by substrate addition measured after 15 mins by... | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Mus musculus) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of LTA4H in C57BL/6 mouse assessed as reduction in LTB4 production pre-incubated for 30 mins before 5-(methylamino)-2-({(2R,3R,6S,8S,9R,11... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50284336 (CHEMBL4174653) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOA using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 15 mins by ... | Eur J Med Chem 143: 33-47 (2018) Article DOI: 10.1016/j.ejmech.2017.08.025 BindingDB Entry DOI: 10.7270/Q2C53PC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50105330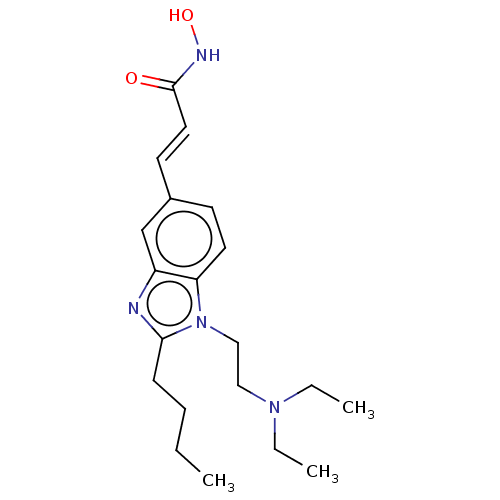 (CHEMBL1851943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H aminopeptidase activity expressed in Escherichia coli BL21 (DE3) pLysS assessed as formation of p-NA from Ala-p... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM207629 (US9265734, R01 | US9796664, Compound R01) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24624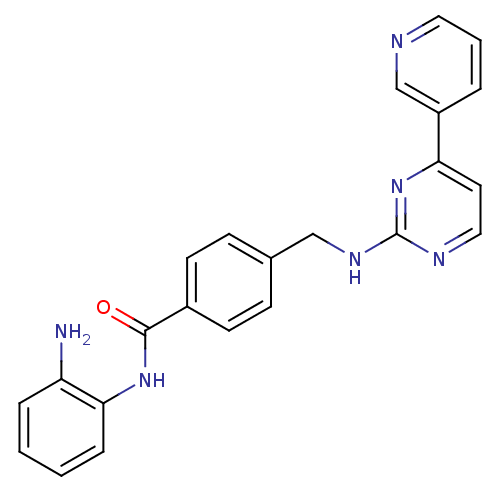 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H aminopeptidase activity expressed in Escherichia coli BL21 (DE3) pLysS assessed as formation of p-NA from Ala-p... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM22449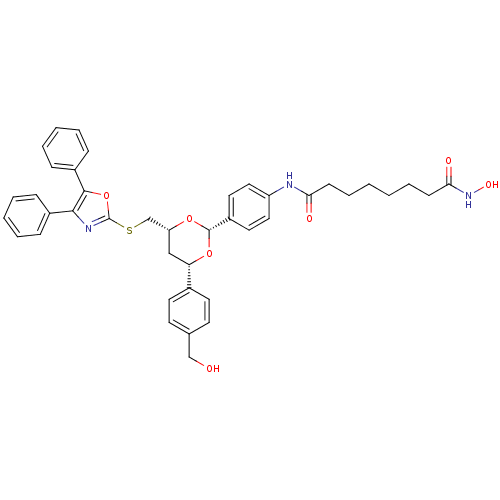 (CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Almond | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM25150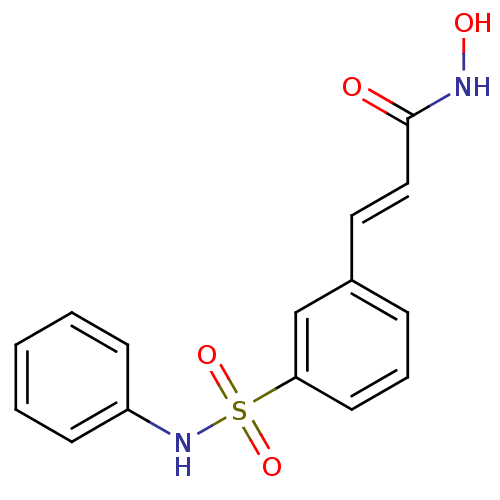 ((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H aminopeptidase activity expressed in Escherichia coli BL21 (DE3) pLysS assessed as formation of p-NA from Ala-p... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H aminopeptidase activity expressed in Escherichia coli BL21 (DE3) pLysS assessed as formation of p-NA from Ala-p... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Almond | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H aminopeptidase activity expressed in Escherichia coli BL21 (DE3) pLysS assessed as formation of p-NA from Ala-p... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50439674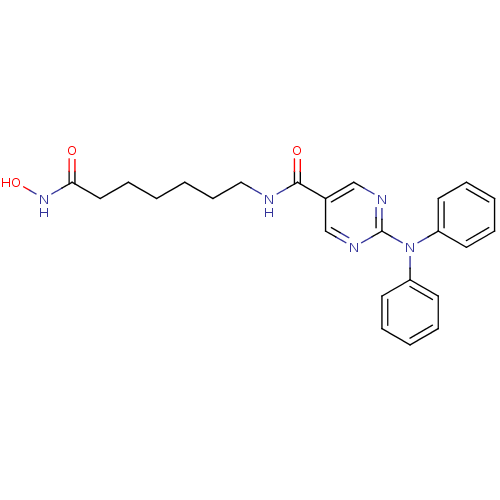 (RICOLINOSTAT | US10858323, Compound 2 | US11207431...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50236887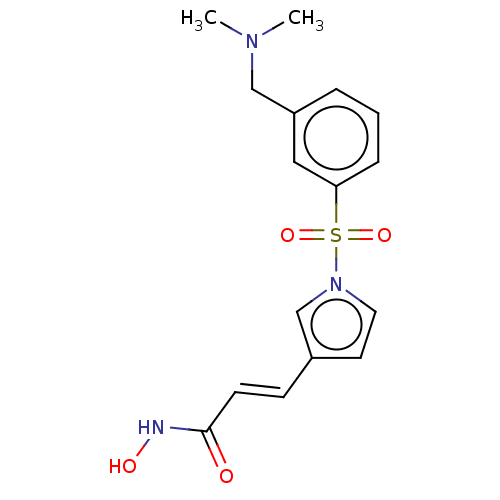 (CHEMBL4082369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50307768 (7-(4-(3-Ethynylphenylamino)-7-methoxyquinazolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50105329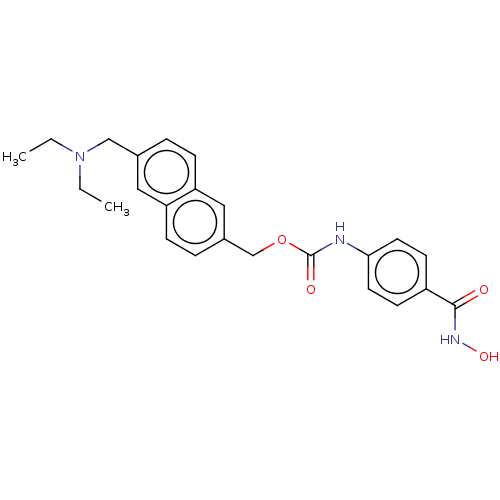 (CHEMBL1213492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50009834 (2-Propylvaleric acid sodium salt | CHEMBL433 | Dep...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM25150 ((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Almond | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H Epoxide Hydrolase expressed in Escherichia coli BL21 (DE3) pLysS preincubated for 10 mins followed by addition ... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM207629 (US9265734, R01 | US9796664, Compound R01) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H aminopeptidase activity expressed in Escherichia coli BL21 (DE3) pLysS assessed as formation of p-NA from Ala-p... | J Med Chem 60: 1817-1828 (2017) Article DOI: 10.1021/acs.jmedchem.6b01507 BindingDB Entry DOI: 10.7270/Q2VM4FJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 106 total ) | Next | Last >> |