 Found 90 hits with Last Name = 'prabhu' and Initial = 'n'
Found 90 hits with Last Name = 'prabhu' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607602

(CHEMBL5220994)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CC(F)(F)C1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607596

(CHEMBL5221053)Show SMILES Clc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607597
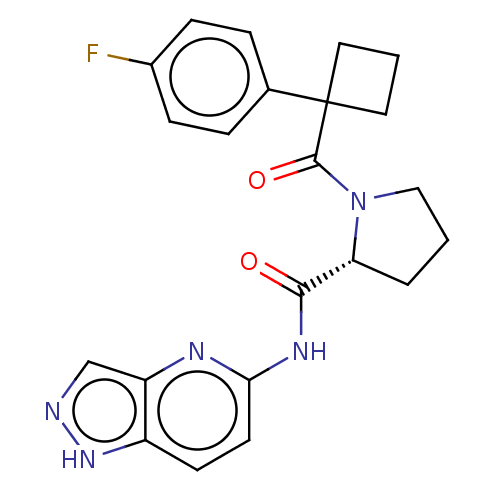
(CHEMBL5219157)Show SMILES Fc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607593
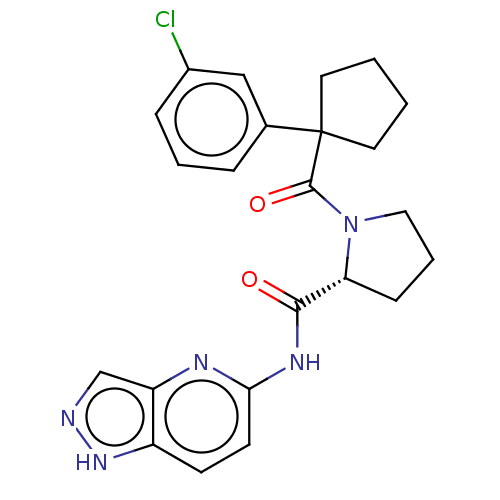
(CHEMBL5219693)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607595

(CHEMBL5219030)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ccc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607601
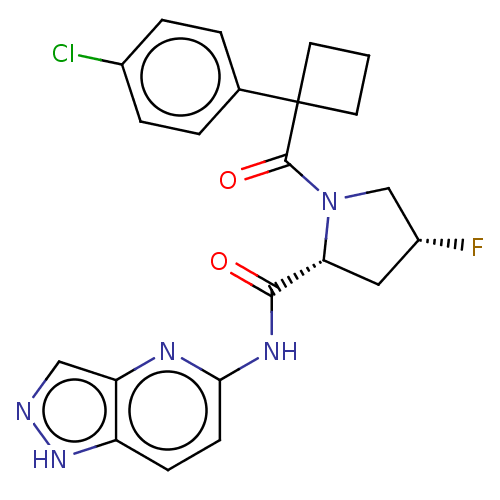
(CHEMBL5219667)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CCC1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607598
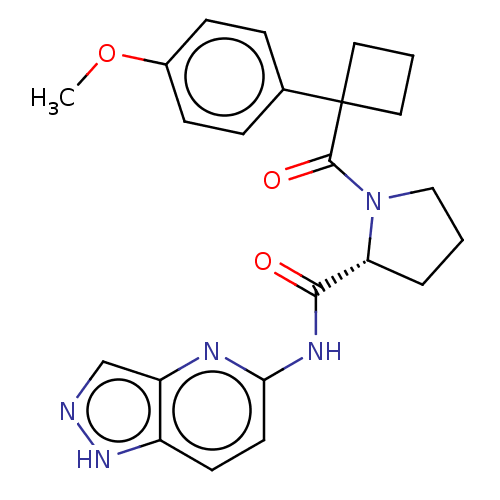
(CHEMBL5220447)Show SMILES COc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607599
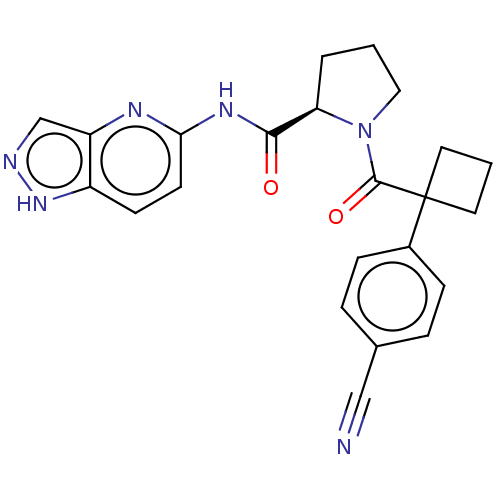
(CHEMBL5220732)Show SMILES O=C(Nc1ccc2[nH]ncc2n1)[C@H]1CCCN1C(=O)C1(CCC1)c1ccc(cc1)C#N |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607589
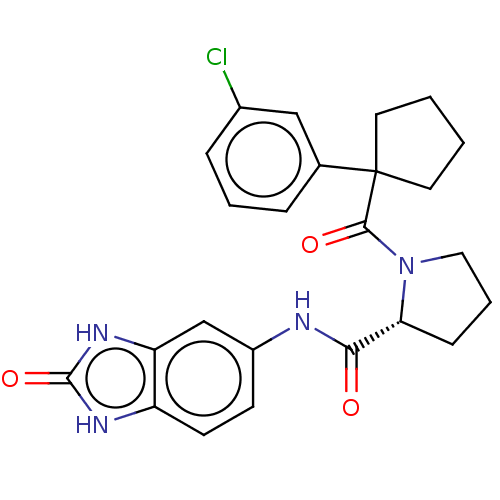
(CHEMBL5219678)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]c(=O)[nH]c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607585
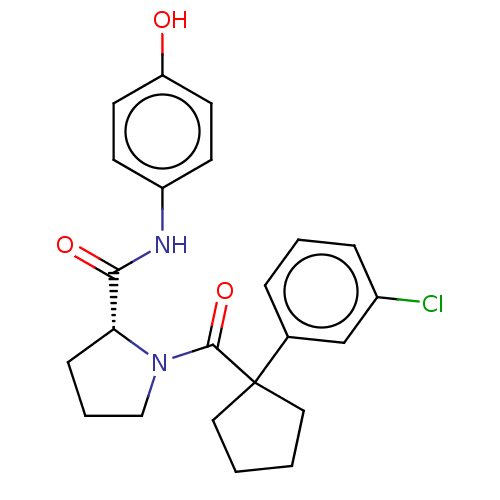
(CHEMBL5219512)Show SMILES Oc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607586
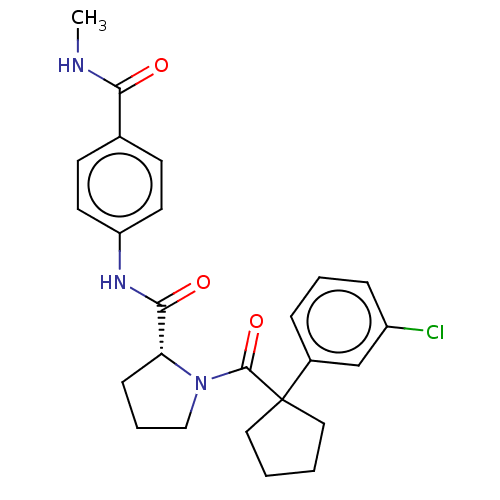
(CHEMBL5221030)Show SMILES CNC(=O)c1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607588
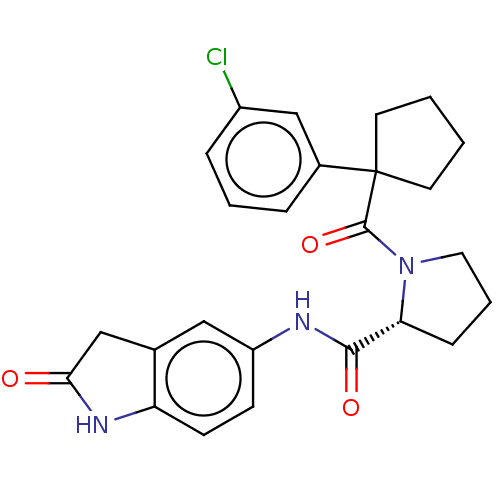
(CHEMBL5220546)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2NC(=O)Cc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607605
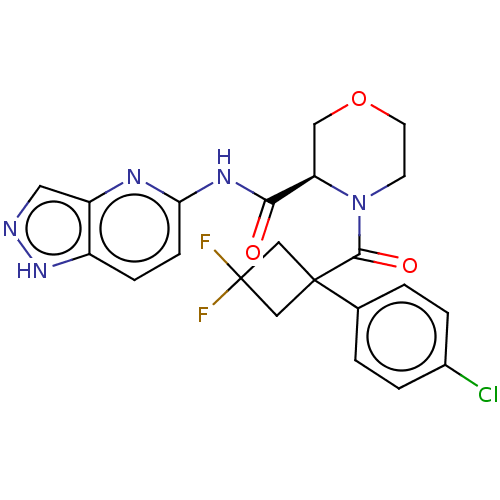
(CHEMBL5219177)Show SMILES FC1(F)CC(C1)(C(=O)N1CCOC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607590
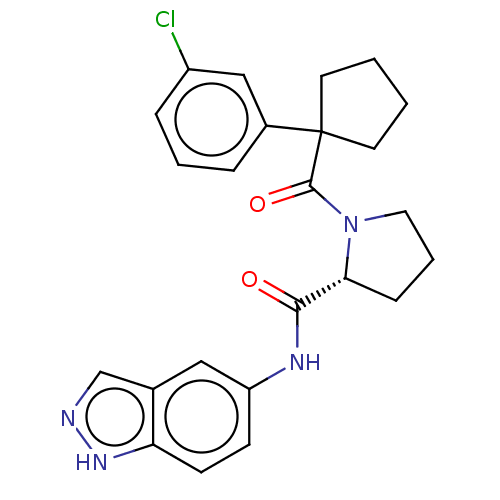
(CHEMBL5220332)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607592
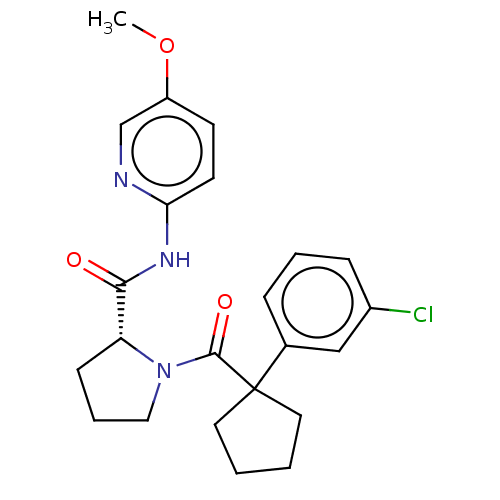
(CHEMBL5219466)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)nc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514606
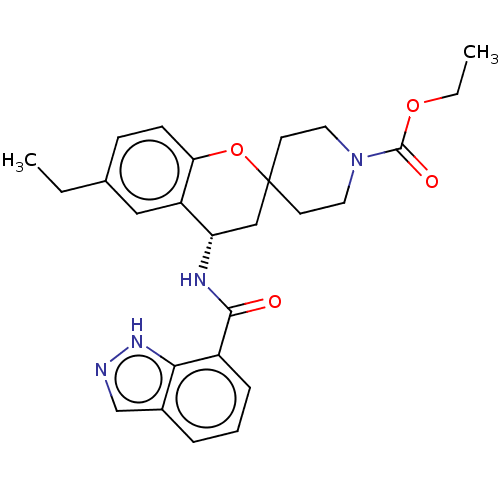
(CHEMBL4517539)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(CC)ccc1O2 |r| Show InChI InChI=1S/C26H30N4O4/c1-3-17-8-9-22-20(14-17)21(28-24(31)19-7-5-6-18-16-27-29-23(18)19)15-26(34-22)10-12-30(13-11-26)25(32)33-4-2/h5-9,14,16,21H,3-4,10-13,15H2,1-2H3,(H,27,29)(H,28,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514614
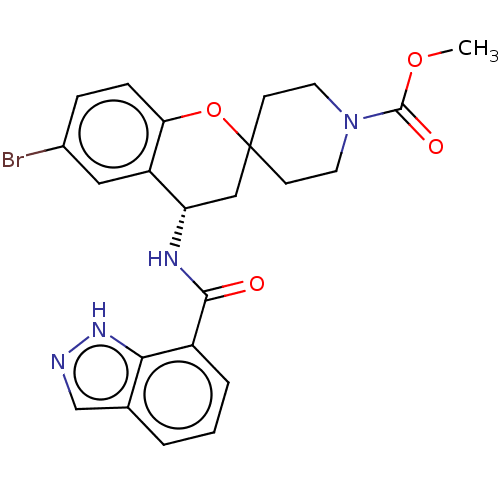
(CHEMBL4533779)Show SMILES COC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C23H23BrN4O4/c1-31-22(30)28-9-7-23(8-10-28)12-18(17-11-15(24)5-6-19(17)32-23)26-21(29)16-4-2-3-14-13-25-27-20(14)16/h2-6,11,13,18H,7-10,12H2,1H3,(H,25,27)(H,26,29)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514621
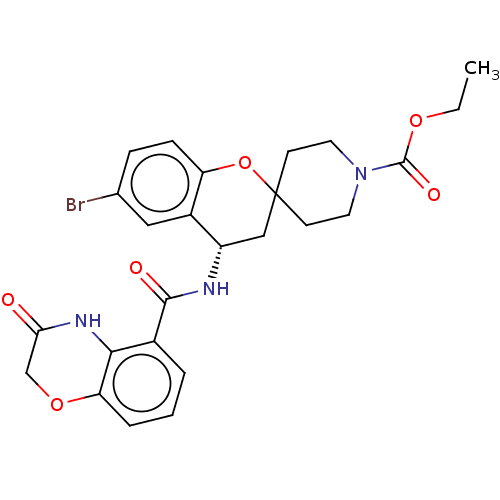
(CHEMBL4443475)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3OCC(=O)Nc13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C25H26BrN3O6/c1-2-33-24(32)29-10-8-25(9-11-29)13-18(17-12-15(26)6-7-19(17)35-25)27-23(31)16-4-3-5-20-22(16)28-21(30)14-34-20/h3-7,12,18H,2,8-11,13-14H2,1H3,(H,27,31)(H,28,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514615
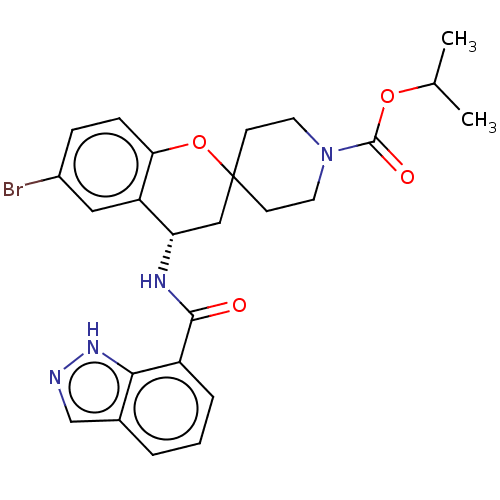
(CHEMBL4520765)Show SMILES CC(C)OC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C25H27BrN4O4/c1-15(2)33-24(32)30-10-8-25(9-11-30)13-20(19-12-17(26)6-7-21(19)34-25)28-23(31)18-5-3-4-16-14-27-29-22(16)18/h3-7,12,14-15,20H,8-11,13H2,1-2H3,(H,27,29)(H,28,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607584
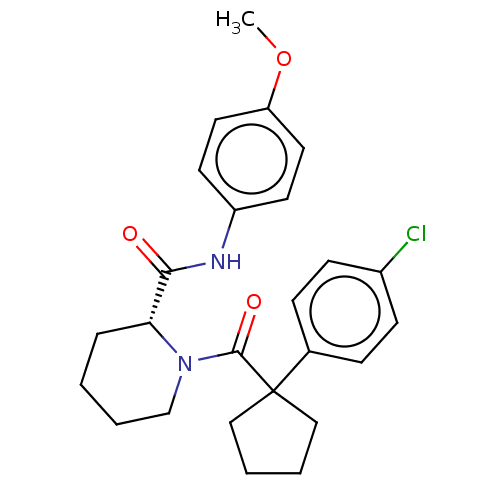
(CHEMBL5221088)Show SMILES COc1ccc(NC(=O)[C@H]2CCCCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514603
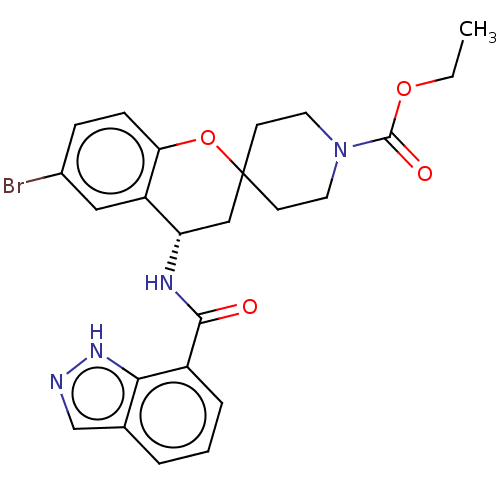
(CHEMBL4550298)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C24H25BrN4O4/c1-2-32-23(31)29-10-8-24(9-11-29)13-19(18-12-16(25)6-7-20(18)33-24)27-22(30)17-5-3-4-15-14-26-28-21(15)17/h3-7,12,14,19H,2,8-11,13H2,1H3,(H,26,28)(H,27,30)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514623
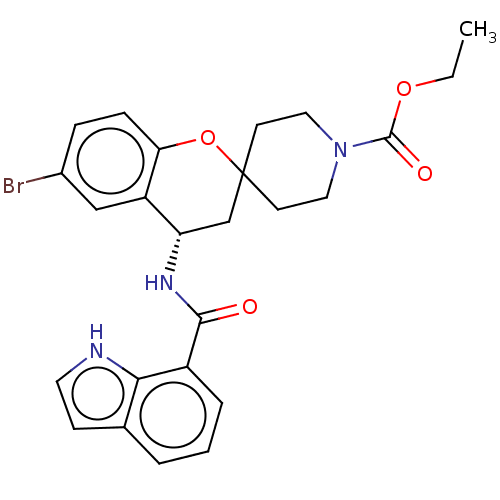
(CHEMBL4457477)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cc[nH]c13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C25H26BrN3O4/c1-2-32-24(31)29-12-9-25(10-13-29)15-20(19-14-17(26)6-7-21(19)33-25)28-23(30)18-5-3-4-16-8-11-27-22(16)18/h3-8,11,14,20,27H,2,9-10,12-13,15H2,1H3,(H,28,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514613
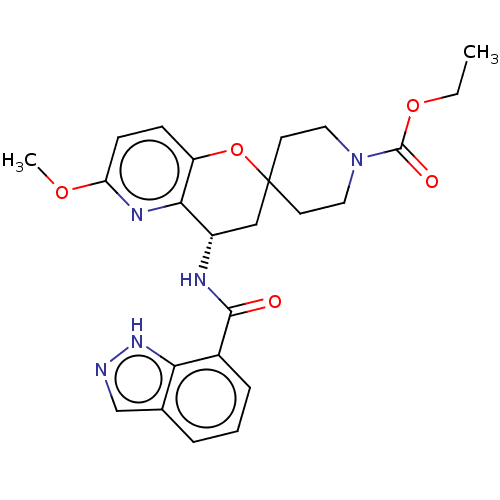
(CHEMBL4459934)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1nc(OC)ccc1O2 |r| Show InChI InChI=1S/C24H27N5O5/c1-3-33-23(31)29-11-9-24(10-12-29)13-17(21-18(34-24)7-8-19(27-21)32-2)26-22(30)16-6-4-5-15-14-25-28-20(15)16/h4-8,14,17H,3,9-13H2,1-2H3,(H,25,28)(H,26,30)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607577
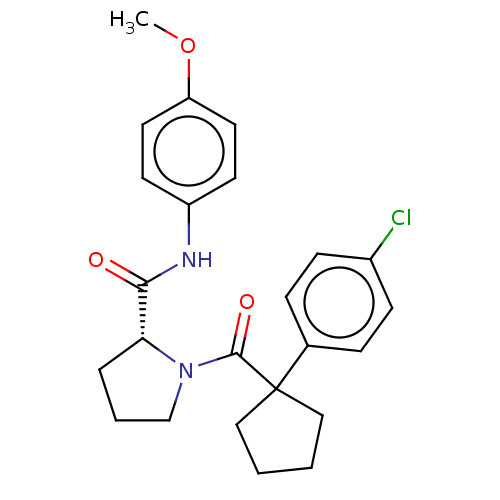
(CHEMBL5219851)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514603

(CHEMBL4550298)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C24H25BrN4O4/c1-2-32-23(31)29-10-8-24(9-11-29)13-19(18-12-16(25)6-7-20(18)33-24)27-22(30)17-5-3-4-15-14-26-28-21(15)17/h3-7,12,14,19H,2,8-11,13H2,1H3,(H,26,28)(H,27,30)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514625
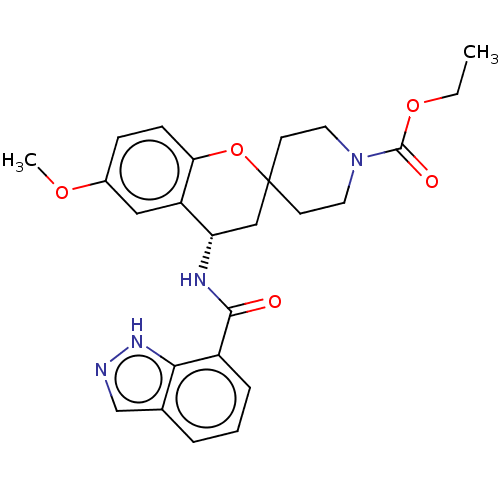
(CHEMBL4521139)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(OC)ccc1O2 |r| Show InChI InChI=1S/C25H28N4O5/c1-3-33-24(31)29-11-9-25(10-12-29)14-20(19-13-17(32-2)7-8-21(19)34-25)27-23(30)18-6-4-5-16-15-26-28-22(16)18/h4-8,13,15,20H,3,9-12,14H2,1-2H3,(H,26,28)(H,27,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514620
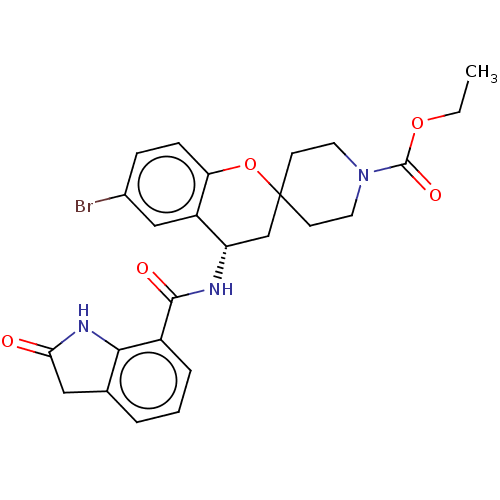
(CHEMBL4529084)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3CC(=O)Nc13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C25H26BrN3O5/c1-2-33-24(32)29-10-8-25(9-11-29)14-19(18-13-16(26)6-7-20(18)34-25)27-23(31)17-5-3-4-15-12-21(30)28-22(15)17/h3-7,13,19H,2,8-12,14H2,1H3,(H,27,31)(H,28,30)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607604
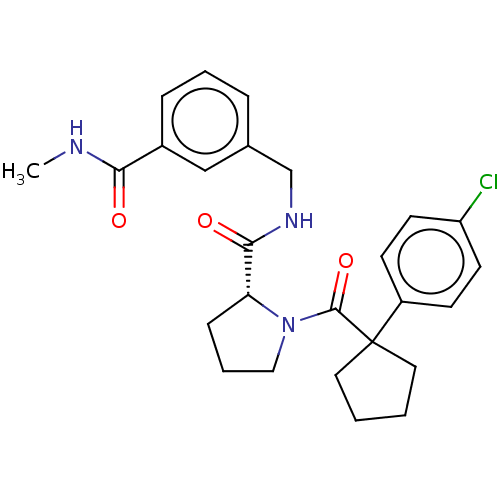
(CHEMBL5219712)Show SMILES CNC(=O)c1cccc(CNC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607580
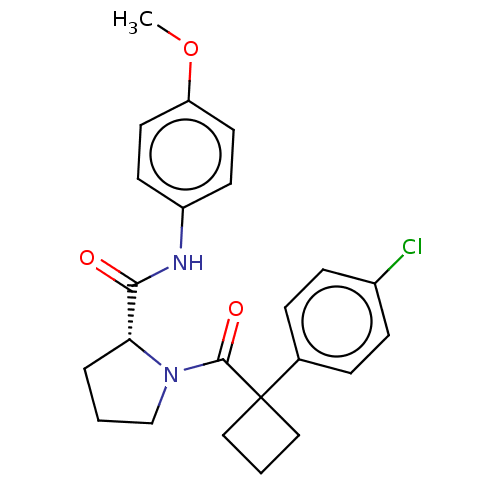
(CHEMBL5218875)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514630
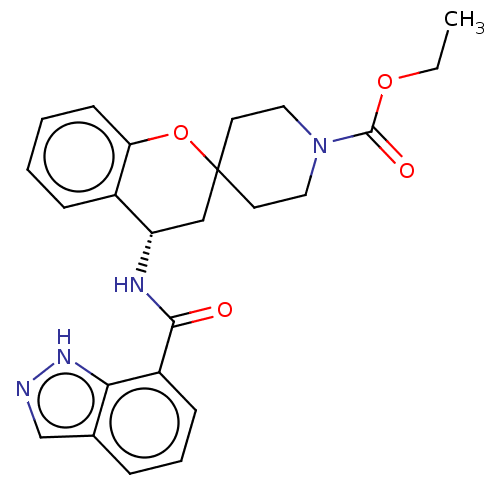
(CHEMBL4463872)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1ccccc1O2 |r| Show InChI InChI=1S/C24H26N4O4/c1-2-31-23(30)28-12-10-24(11-13-28)14-19(17-7-3-4-9-20(17)32-24)26-22(29)18-8-5-6-16-15-25-27-21(16)18/h3-9,15,19H,2,10-14H2,1H3,(H,25,27)(H,26,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514631
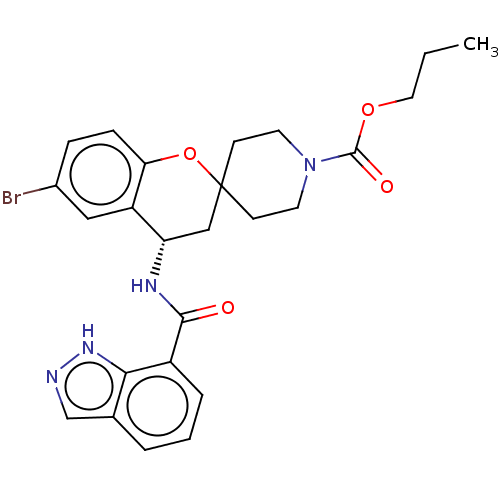
(CHEMBL4456038)Show SMILES CCCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C25H27BrN4O4/c1-2-12-33-24(32)30-10-8-25(9-11-30)14-20(19-13-17(26)6-7-21(19)34-25)28-23(31)18-5-3-4-16-15-27-29-22(16)18/h3-7,13,15,20H,2,8-12,14H2,1H3,(H,27,29)(H,28,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607594
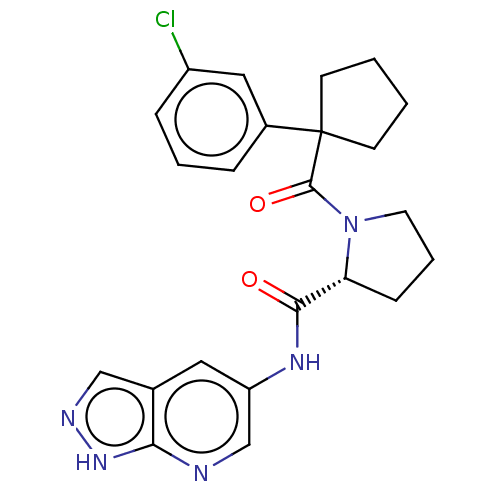
(CHEMBL5219204)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1cnc2[nH]ncc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514617
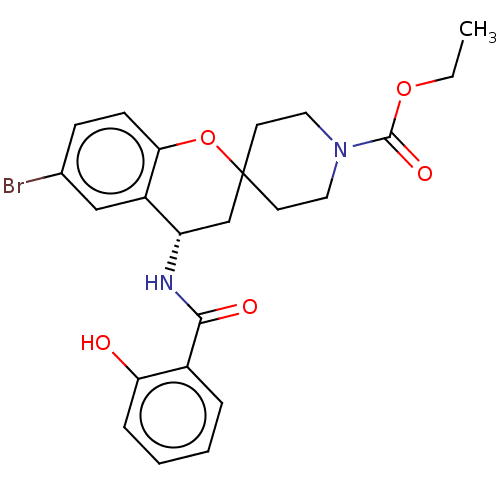
(CHEMBL4447760)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1ccccc1O)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C23H25BrN2O5/c1-2-30-22(29)26-11-9-23(10-12-26)14-18(17-13-15(24)7-8-20(17)31-23)25-21(28)16-5-3-4-6-19(16)27/h3-8,13,18,27H,2,9-12,14H2,1H3,(H,25,28)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514632
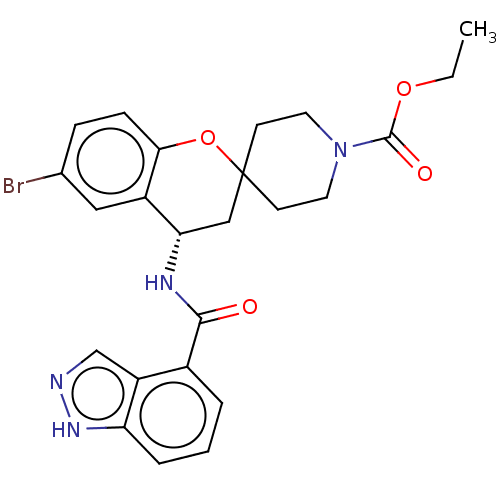
(CHEMBL4448431)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3[nH]ncc13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C24H25BrN4O4/c1-2-32-23(31)29-10-8-24(9-11-29)13-20(17-12-15(25)6-7-21(17)33-24)27-22(30)16-4-3-5-19-18(16)14-26-28-19/h3-7,12,14,20H,2,8-11,13H2,1H3,(H,26,28)(H,27,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514624
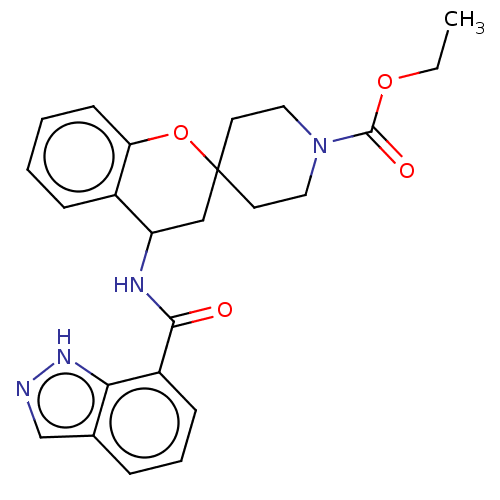
(CHEMBL4476353)Show SMILES CCOC(=O)N1CCC2(CC1)CC(NC(=O)c1cccc3cn[nH]c13)c1ccccc1O2 Show InChI InChI=1S/C24H26N4O4/c1-2-31-23(30)28-12-10-24(11-13-28)14-19(17-7-3-4-9-20(17)32-24)26-22(29)18-8-5-6-16-15-25-27-21(16)18/h3-9,15,19H,2,10-14H2,1H3,(H,25,27)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514612

(CHEMBL4475838)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(ccc1O2)C#N |r| Show InChI InChI=1S/C25H25N5O4/c1-2-33-24(32)30-10-8-25(9-11-30)13-20(19-12-16(14-26)6-7-21(19)34-25)28-23(31)18-5-3-4-17-15-27-29-22(17)18/h3-7,12,15,20H,2,8-11,13H2,1H3,(H,27,29)(H,28,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607591
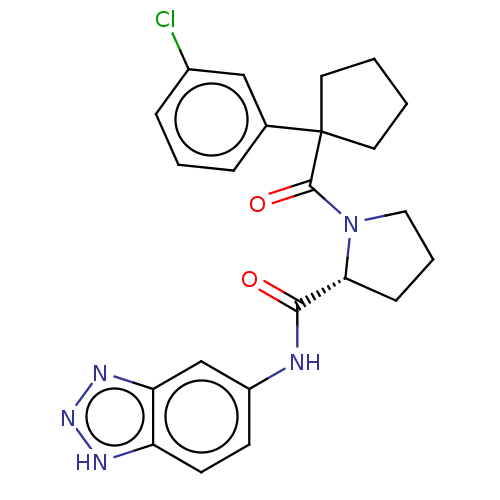
(CHEMBL5219472)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]nnc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607581
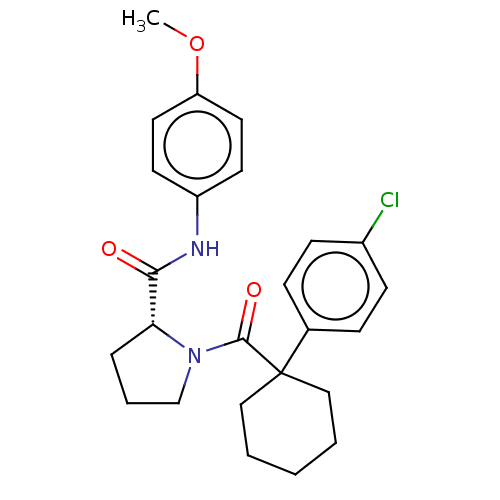
(CHEMBL5221117)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514626
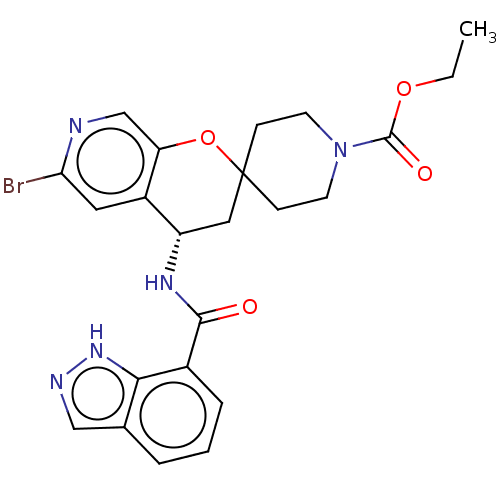
(CHEMBL4474052)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(Br)ncc1O2 |r| Show InChI InChI=1S/C23H24BrN5O4/c1-2-32-22(31)29-8-6-23(7-9-29)11-17(16-10-19(24)25-13-18(16)33-23)27-21(30)15-5-3-4-14-12-26-28-20(14)15/h3-5,10,12-13,17H,2,6-9,11H2,1H3,(H,26,28)(H,27,30)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514618
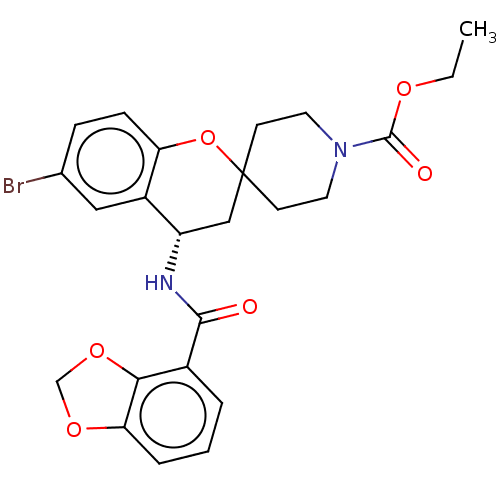
(CHEMBL4541737)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3OCOc13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C24H25BrN2O6/c1-2-30-23(29)27-10-8-24(9-11-27)13-18(17-12-15(25)6-7-19(17)33-24)26-22(28)16-4-3-5-20-21(16)32-14-31-20/h3-7,12,18H,2,8-11,13-14H2,1H3,(H,26,28)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514608
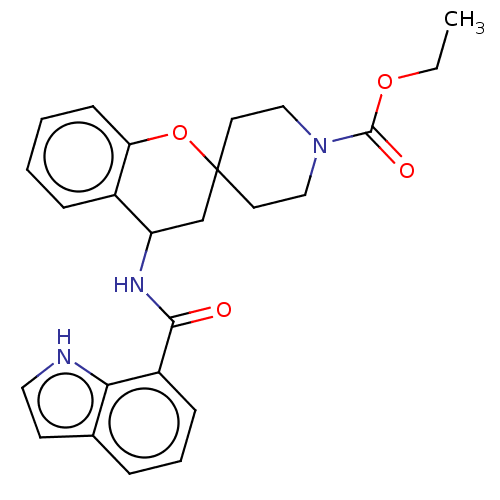
(CHEMBL4467532)Show SMILES CCOC(=O)N1CCC2(CC1)CC(NC(=O)c1cccc3cc[nH]c13)c1ccccc1O2 Show InChI InChI=1S/C25H27N3O4/c1-2-31-24(30)28-14-11-25(12-15-28)16-20(18-7-3-4-9-21(18)32-25)27-23(29)19-8-5-6-17-10-13-26-22(17)19/h3-10,13,20,26H,2,11-12,14-16H2,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514616
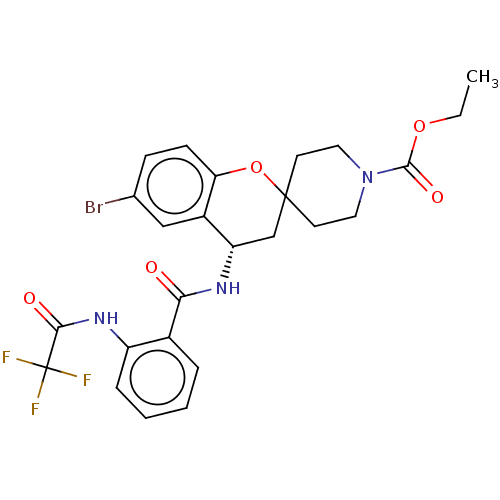
(CHEMBL4483789)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1ccccc1NC(=O)C(F)(F)F)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C25H25BrF3N3O5/c1-2-36-23(35)32-11-9-24(10-12-32)14-19(17-13-15(26)7-8-20(17)37-24)30-21(33)16-5-3-4-6-18(16)31-22(34)25(27,28)29/h3-8,13,19H,2,9-12,14H2,1H3,(H,30,33)(H,31,34)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514628
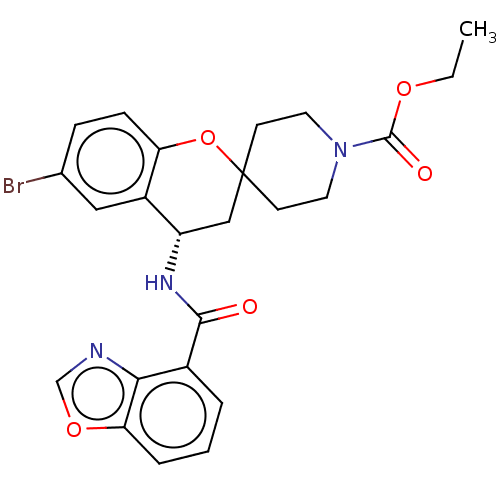
(CHEMBL4442192)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3ocnc13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C24H24BrN3O5/c1-2-31-23(30)28-10-8-24(9-11-28)13-18(17-12-15(25)6-7-19(17)33-24)27-22(29)16-4-3-5-20-21(16)26-14-32-20/h3-7,12,14,18H,2,8-11,13H2,1H3,(H,27,29)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607583
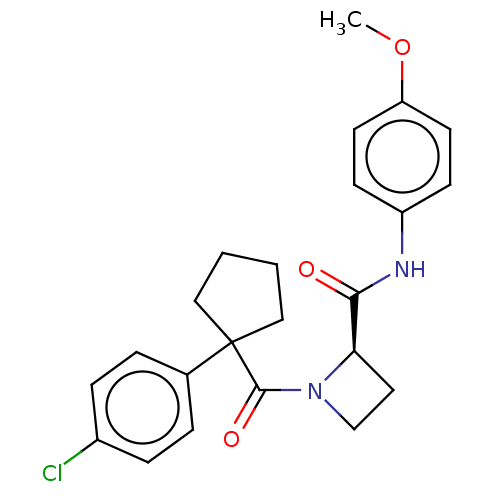
(CHEMBL5218882)Show SMILES COc1ccc(NC(=O)[C@H]2CCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514629

(CHEMBL4529203)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3[nH]c(=O)[nH]c13)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C24H25BrN4O5/c1-2-33-23(32)29-10-8-24(9-11-29)13-18(16-12-14(25)6-7-19(16)34-24)26-21(30)15-4-3-5-17-20(15)28-22(31)27-17/h3-7,12,18H,2,8-11,13H2,1H3,(H,26,30)(H2,27,28,31)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607582
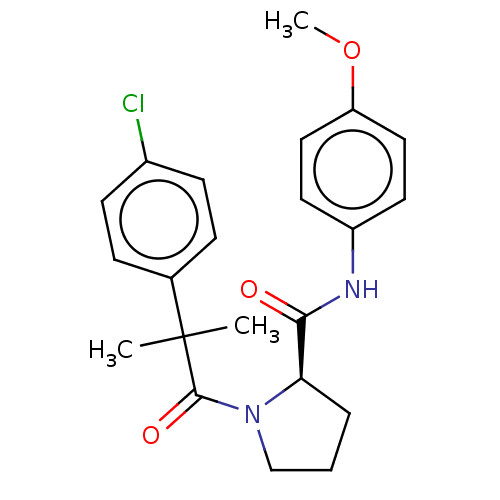
(CHEMBL5220135)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C(C)(C)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514611
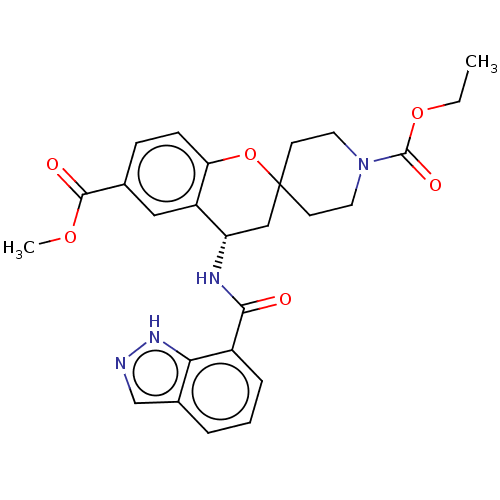
(CHEMBL4543807)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(ccc1O2)C(=O)OC |r| Show InChI InChI=1S/C26H28N4O6/c1-3-35-25(33)30-11-9-26(10-12-30)14-20(19-13-16(24(32)34-2)7-8-21(19)36-26)28-23(31)18-6-4-5-17-15-27-29-22(17)18/h4-8,13,15,20H,3,9-12,14H2,1-2H3,(H,27,29)(H,28,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514604
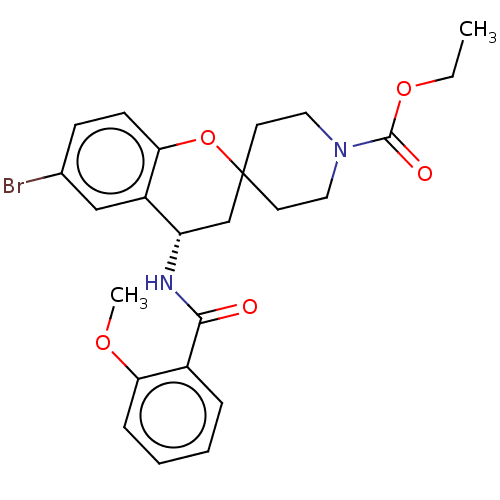
(CHEMBL4441700)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1ccccc1OC)c1cc(Br)ccc1O2 |r| Show InChI InChI=1S/C24H27BrN2O5/c1-3-31-23(29)27-12-10-24(11-13-27)15-19(18-14-16(25)8-9-21(18)32-24)26-22(28)17-6-4-5-7-20(17)30-2/h4-9,14,19H,3,10-13,15H2,1-2H3,(H,26,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data