

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398475 (CHEMBL2179131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398472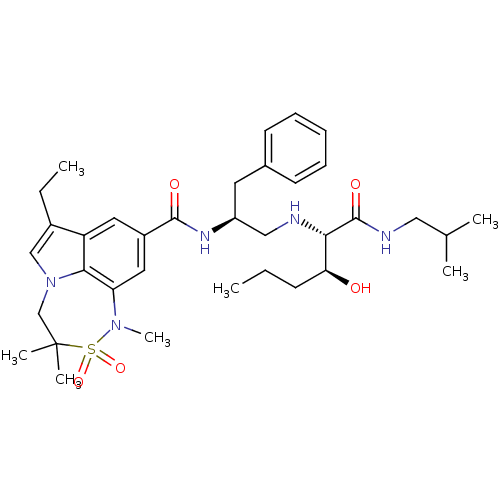 (CHEMBL2179138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16254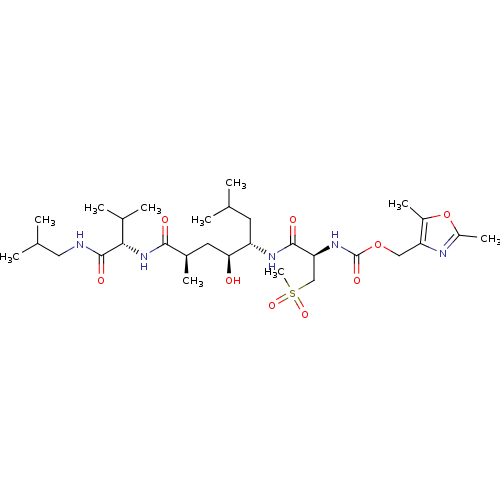 ((2,5-dimethyl-1,3-oxazol-4-yl)methyl N-[(1R)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of BACE1 | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398473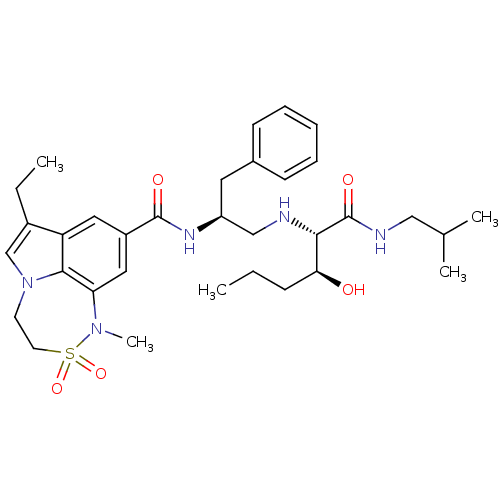 (CHEMBL2179137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398471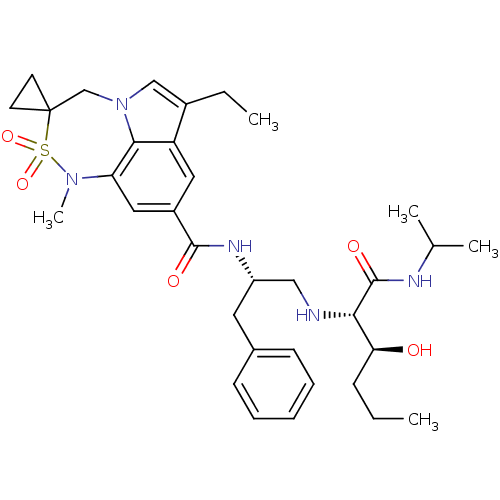 (CHEMBL2179140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of BACE1 | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50524365 (CHEMBL4579211) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Scientific and Innovative Research (AcSIR) Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant human H3 receptor expressed in HEK293T cells measured after 90 mins by liquid scintillat... | J Med Chem 62: 4638-4655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00241 BindingDB Entry DOI: 10.7270/Q23T9MN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50524374 (CHEMBL4453810) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Scientific and Innovative Research (AcSIR) Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant human H3 receptor expressed in HEK293T cells measured after 90 mins by liquid scintillat... | J Med Chem 62: 4638-4655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00241 BindingDB Entry DOI: 10.7270/Q23T9MN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398478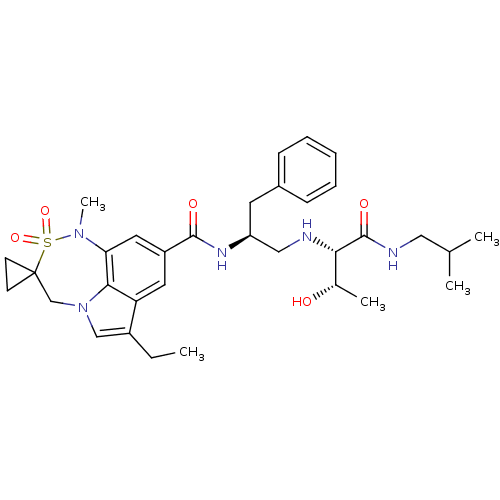 (CHEMBL2179139) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398474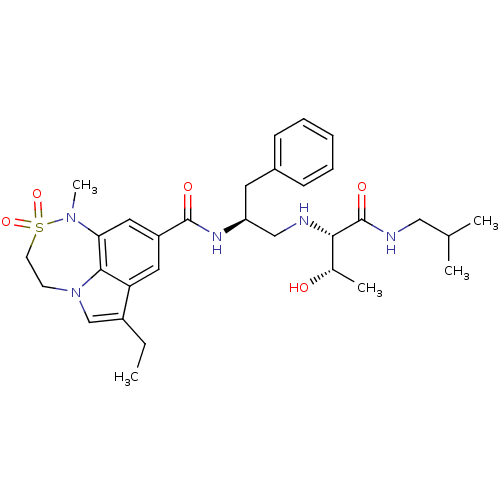 (CHEMBL2179136) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50398472 (CHEMBL2179138) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE2 | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398480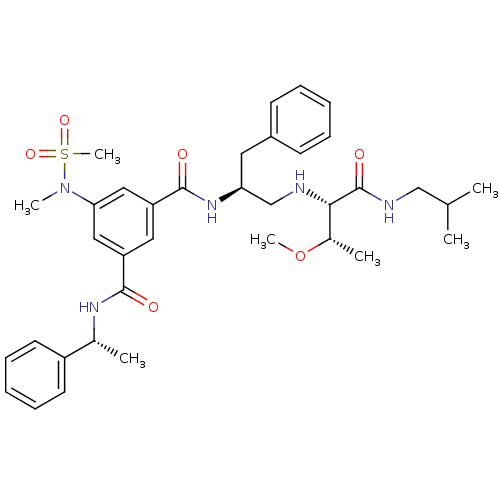 (CHEMBL2179134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398476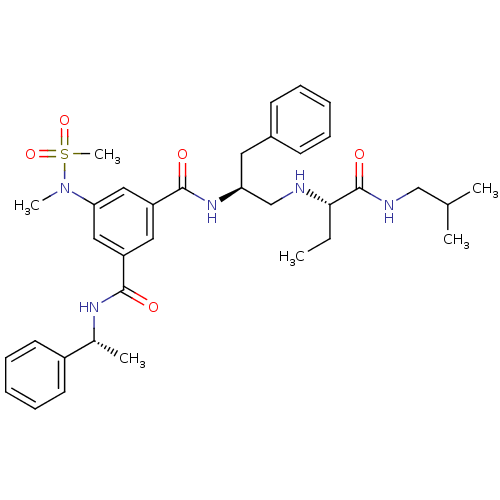 (CHEMBL2179130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398477 (CHEMBL2179132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50398475 (CHEMBL2179131) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE2 | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50398472 (CHEMBL2179138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cathepsin D | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50398473 (CHEMBL2179137) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cathepsin D | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398479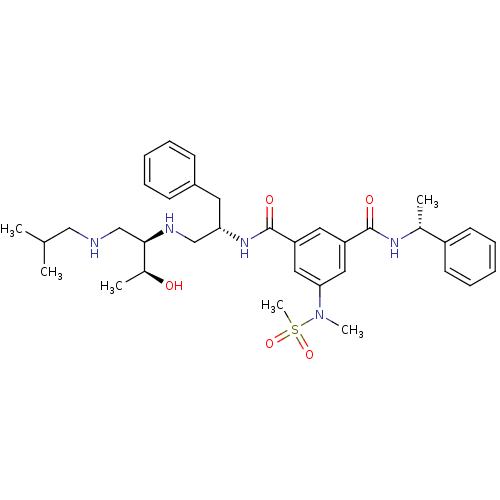 (CHEMBL2179135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398481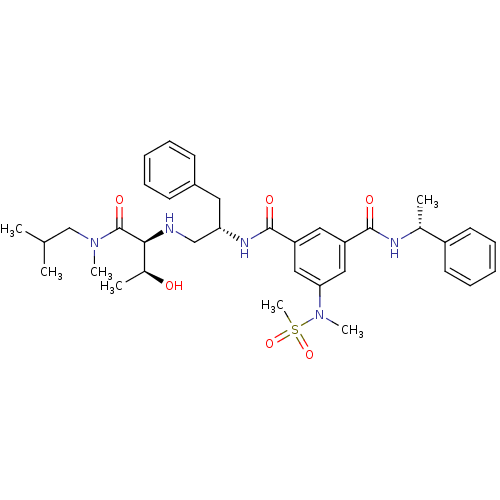 (CHEMBL2179133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50398476 (CHEMBL2179130) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE2 | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50398471 (CHEMBL2179140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cathepsin D | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50398475 (CHEMBL2179131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cathepsin D | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50398474 (CHEMBL2179136) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cathepsin D | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50398476 (CHEMBL2179130) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cathepsin D | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456266 (((R)-1-(5-(((2-hydroxy-2- (4-methyl-1-oxo-1,3- dih...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2), 1 Mg... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50594991 (CHEMBL415320) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114199 BindingDB Entry DOI: 10.7270/Q2CN77X7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50594992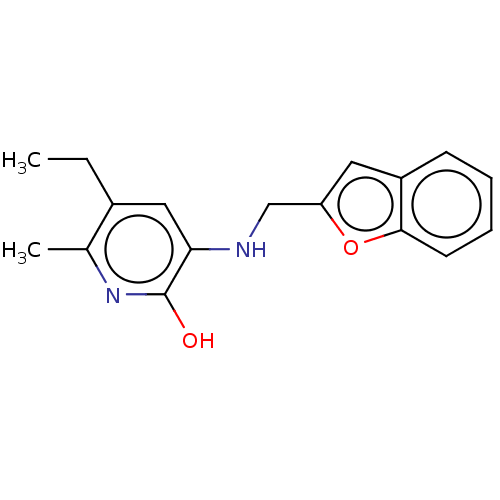 (CHEMBL316785) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114199 BindingDB Entry DOI: 10.7270/Q2CN77X7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456266 (((R)-1-(5-(((2-hydroxy-2- (4-methyl-1-oxo-1,3- dih...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Centre, Alkem Laboratories Ltd. , Peenya Ind. Area, 3rd Stage, Bangalore 560 058, India. Curated by ChEMBL | Assay Description Agonist activity at recombinant human KOR expressed in HEK293T cells assessed as inhibition of forskolin-stimulated cAMP level preincubated for 15 to... | J Med Chem 60: 6733-6750 (2017) Article DOI: 10.1021/acs.jmedchem.7b00643 BindingDB Entry DOI: 10.7270/Q2VT1VK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Centre, Alkem Laboratories Ltd. , Peenya Ind. Area, 3rd Stage, Bangalore 560 058, India. Curated by ChEMBL | Assay Description Agonist activity at recombinant human KOR expressed in HEK293T cells assessed as inhibition of forskolin-stimulated cAMP level preincubated for 15 to... | J Med Chem 60: 6733-6750 (2017) Article DOI: 10.1021/acs.jmedchem.7b00643 BindingDB Entry DOI: 10.7270/Q2VT1VK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398475 (CHEMBL2179131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of BACE1 in human neuroblastoma cells | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456149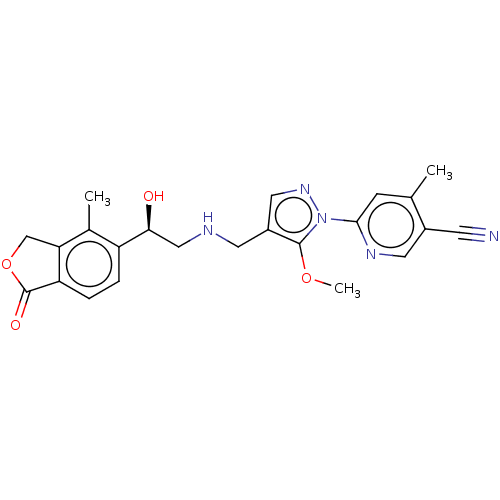 (US10723723, Example 35) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456226 ((R)-5'-(((2-hydroxy-2-(4- methyl-1-oxo-1,3- dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2), 1 Mg... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456268 ((R)-1-(5-(((2-hydroxy-2- (4-methyl-1-oxo-1,3- dihy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2), 1 Mg... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456260 ((R)-6-(4-(((2-hydroxy-2- (4-methyl-1-oxo-1,3- dihy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50231937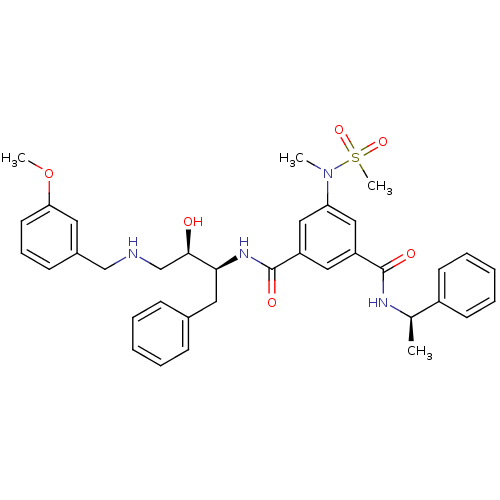 (CHEMBL403268 | N-{(1S,2R)-1-benzyl-2-hydroxy-3-[(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of BACE1 in human neuroblastoma cells | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50088381 (ADL 8-2698 | Alvimopan | Entereg) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Centre, Alkem Laboratories Ltd. , Peenya Ind. Area, 3rd Stage, Bangalore 560 058, India. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human MOR expressed in HEK293T cells assessed as reduction in DAMGO-induced inhibition of forskolin-stimulated cAM... | J Med Chem 60: 6733-6750 (2017) Article DOI: 10.1021/acs.jmedchem.7b00643 BindingDB Entry DOI: 10.7270/Q2VT1VK0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50088381 (ADL 8-2698 | Alvimopan | Entereg) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Centre, Alkem Laboratories Ltd. , Peenya Ind. Area, 3rd Stage, Bangalore 560 058, India. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human MOR expressed in HEK293T cells assessed as reduction in DAMGO-induced inhibition of forskolin-stimulated cAM... | J Med Chem 60: 6733-6750 (2017) Article DOI: 10.1021/acs.jmedchem.7b00643 BindingDB Entry DOI: 10.7270/Q2VT1VK0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456268 ((R)-1-(5-(((2-hydroxy-2- (4-methyl-1-oxo-1,3- dihy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456264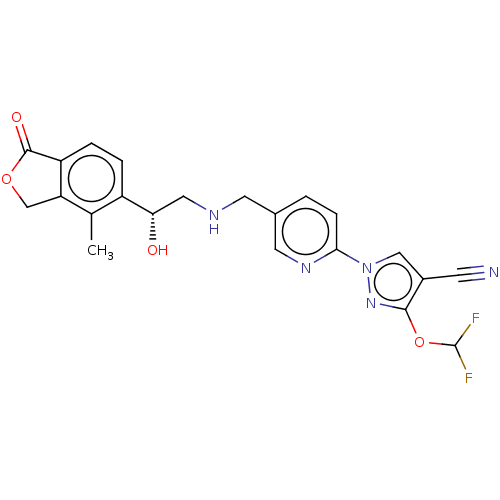 ((R)-3-(difluoromethoxy)- 1-(5-(((2-hydroxy-2-(4- m...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456277 (6-(4-(((1-hydroxy-1-(4- methyl-1-oxo-1,3- dihydroi...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM423209 ((R)-4-methyl-5-(4-((6- (4-methyl-1H-imidazol- 1-yl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgC... | US Patent US10501449 (2019) BindingDB Entry DOI: 10.7270/Q2M32Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM456141 (US10723723, Example 7-I | US10723723, Example 7-II) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2), 1 Mg... | US Patent US10723723 (2020) BindingDB Entry DOI: 10.7270/Q22Z18K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM423206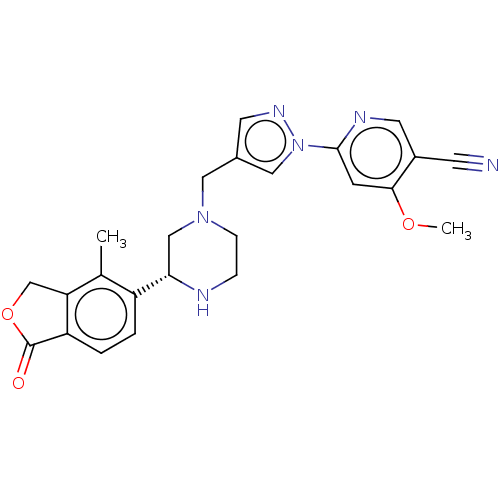 ((R)-4-methoxy-6-(4- ((3-(4-methyl-1-oxo-1,3- dihyd...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgC... | US Patent US10501449 (2019) BindingDB Entry DOI: 10.7270/Q2M32Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM423204 (US10501449, Example 23-I | US10501449, Example 255...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgC... | US Patent US10501449 (2019) BindingDB Entry DOI: 10.7270/Q2M32Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM295330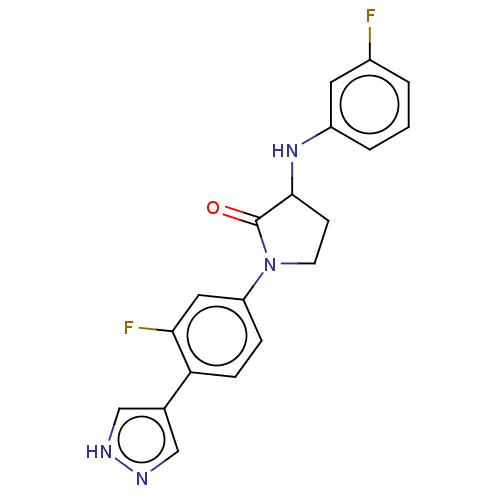 (US10112929, Example 47 | US10112929, Example 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20... | US Patent US10112929 (2018) BindingDB Entry DOI: 10.7270/Q2T72KGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM295332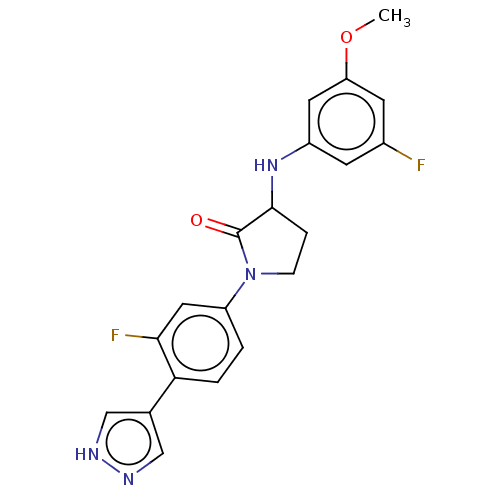 (US10112929, Example 49 | US10112929, Example 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20... | US Patent US10112929 (2018) BindingDB Entry DOI: 10.7270/Q2T72KGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM295332 (US10112929, Example 49 | US10112929, Example 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20... | US Patent US10112929 (2018) BindingDB Entry DOI: 10.7270/Q2T72KGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM295345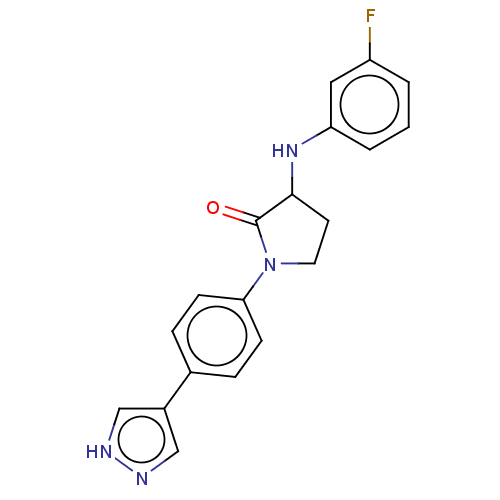 (US10112929, Example 62 | US10112929, Example 91) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20... | US Patent US10112929 (2018) BindingDB Entry DOI: 10.7270/Q2T72KGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM295311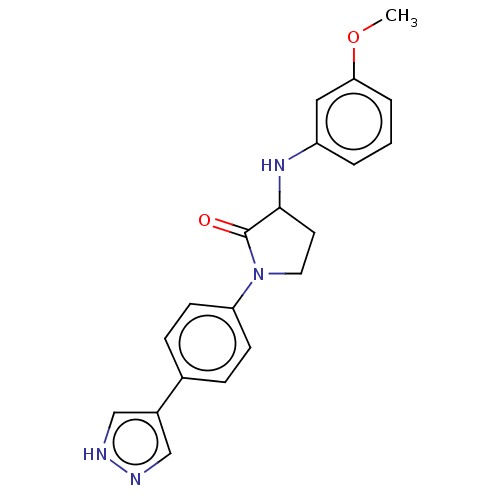 (BDBM295346 | US10112929, Example 28 | US10112929, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20... | US Patent US10112929 (2018) BindingDB Entry DOI: 10.7270/Q2T72KGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1139 total ) | Next | Last >> |