

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50134199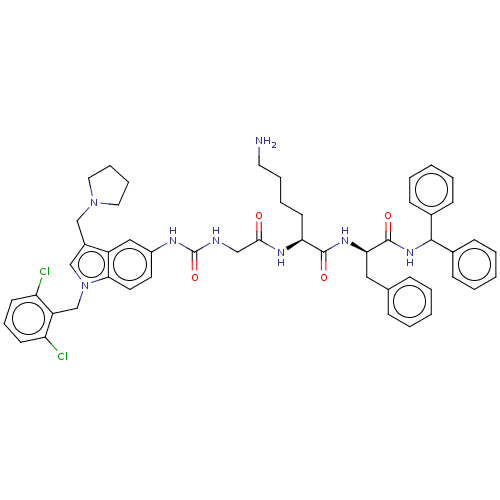 (CHEMBL3735057) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 627 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Displacement of [3H]furoyl-LIGRL-NH2 from human protease-activated receptor 2 in NCTC-2544 cells | Bioorg Med Chem 23: 7717-27 (2015) Article DOI: 10.1016/j.bmc.2015.11.016 BindingDB Entry DOI: 10.7270/Q2VH5QP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50134200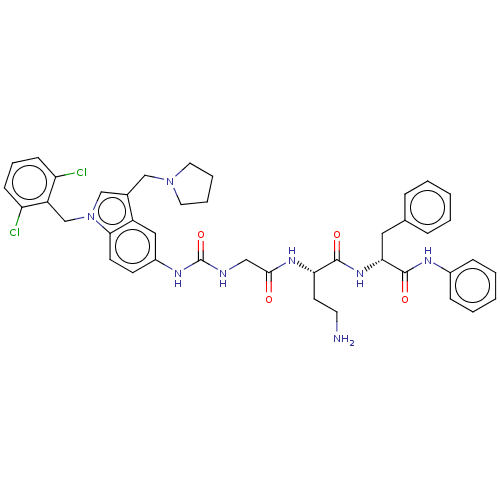 (CHEMBL3735405) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Displacement of [3H]furoyl-LIGRL-NH2 from human protease-activated receptor 2 in NCTC-2544 cells | Bioorg Med Chem 23: 7717-27 (2015) Article DOI: 10.1016/j.bmc.2015.11.016 BindingDB Entry DOI: 10.7270/Q2VH5QP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM24226 (1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of human ERG assessed as reduction in channel current by automated patch-clamp electrophysiology assay | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of human C-RAF measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOA using p-tyramine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50500971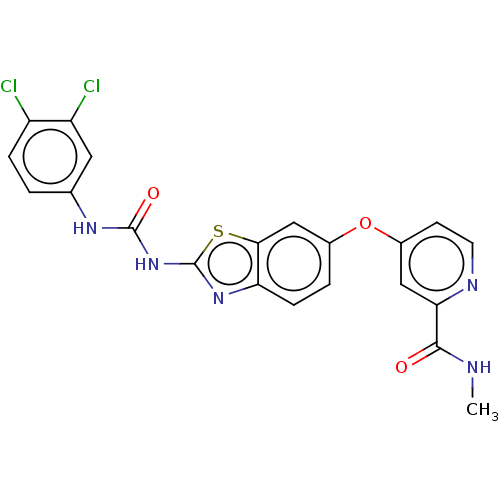 (CHEMBL3798983) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of human C-RAF measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM152636 ((2E)-1-(4-methoxyphenyl)-3-[2-(trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of BRAF V600E mutant (unknown origin) measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM152633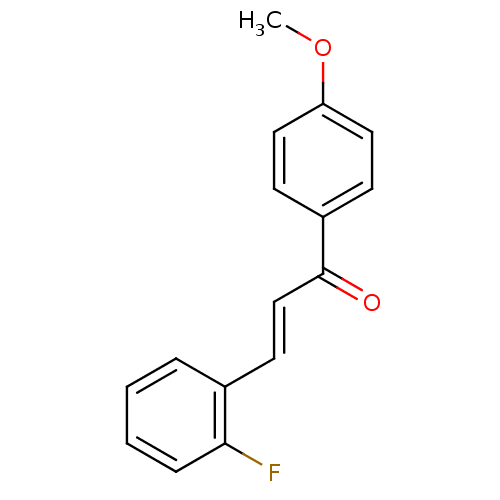 ((2E)-3-(2-fluorophenyl)-1-(4-methoxyphenyl)prop-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117994 (CHEMBL3613158) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117988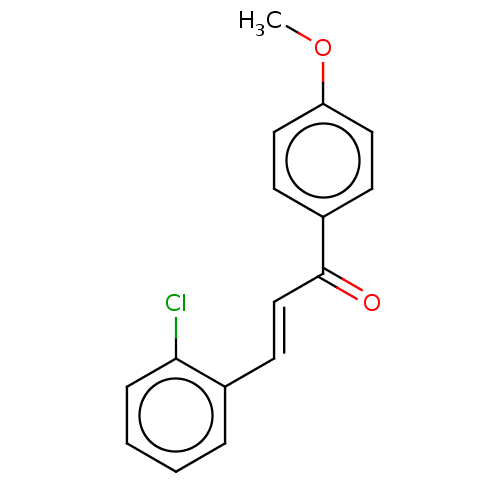 (CHEMBL2013137) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50500971 (CHEMBL3798983) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of BRAF V600E mutant (unknown origin) measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50500969 (CHEMBL3799746) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of human C-RAF measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM19187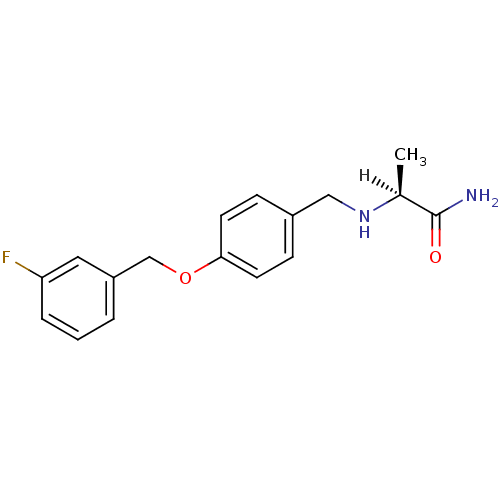 ((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50500969 (CHEMBL3799746) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of BRAF V600E mutant (unknown origin) measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117979 (CHEMBL1802004) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50042986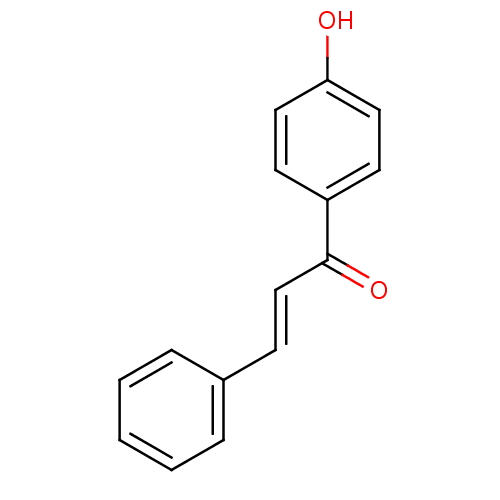 ((E)-1-(4-Hydroxy-phenyl)-3-phenyl-propenone | 1-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM152637 ((2E)-1-(4-methoxyphenyl)-3-[4-(trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118006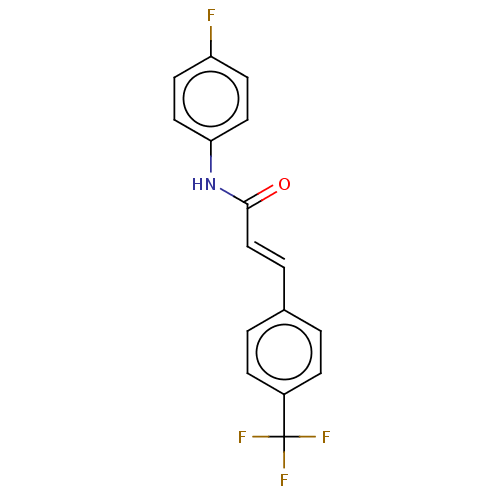 (CHEMBL3613273) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117982 (CHEMBL1801962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118004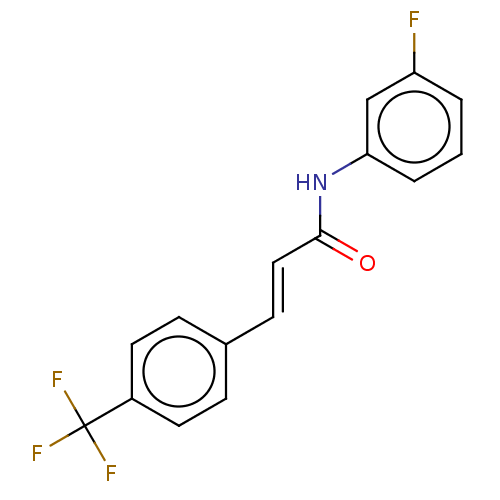 (CHEMBL3613271) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 283 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117990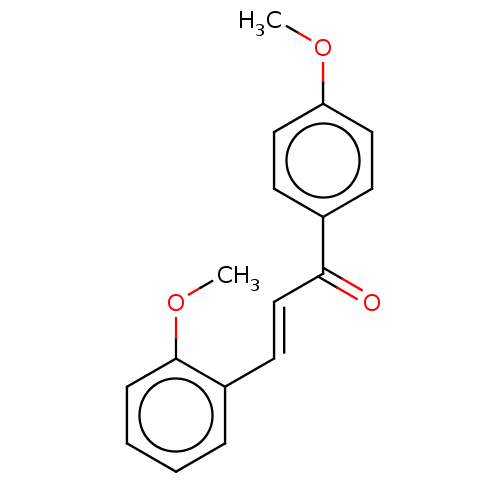 (CHEMBL2236844) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 419 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50141532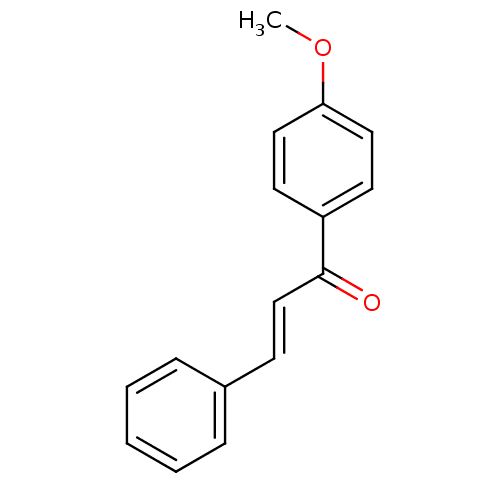 ((E)-1-(4-Methoxy-phenyl)-3-phenyl-propenone | 1-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 471 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50500970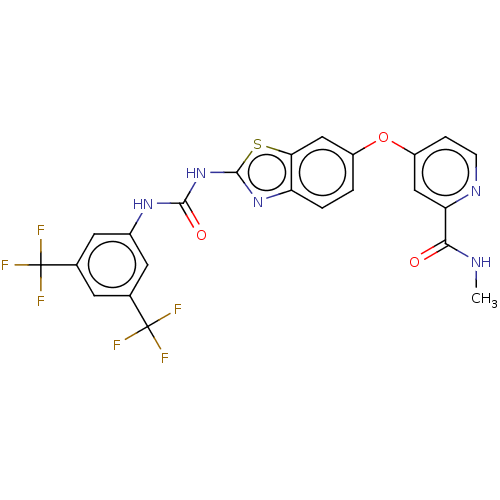 (CHEMBL3797601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 566 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of human C-RAF measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118005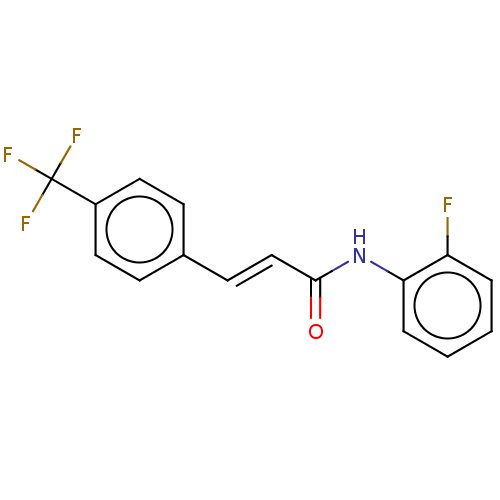 (CHEMBL3613272) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117995 (CHEMBL3260398) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 624 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50134198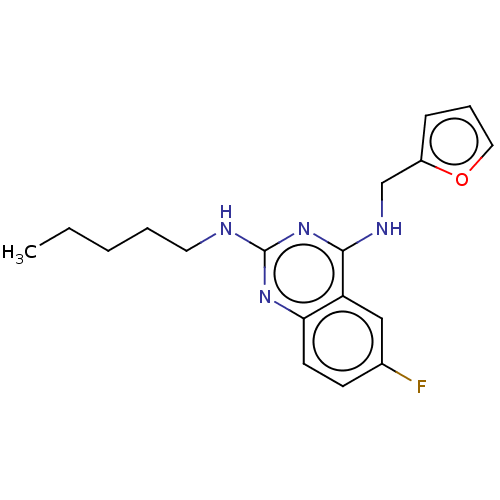 (CHEMBL1684609) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Antagonist activity at protease-activated receptor 2 (unknown origin) expressed in CHO-K1 cells assessed as inhibition of trypsin-induced calcium flu... | Bioorg Med Chem 23: 7717-27 (2015) Article DOI: 10.1016/j.bmc.2015.11.016 BindingDB Entry DOI: 10.7270/Q2VH5QP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50500968 (CHEMBL3799621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 695 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of human C-RAF measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117981 (CHEMBL1801958) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 793 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118011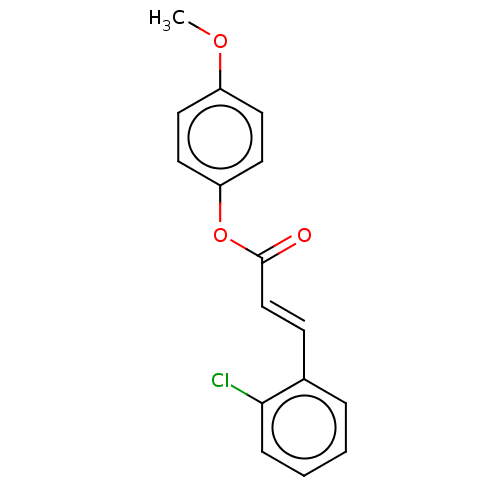 (CHEMBL3613276) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 937 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50500970 (CHEMBL3797601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of BRAF V600E mutant (unknown origin) measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117991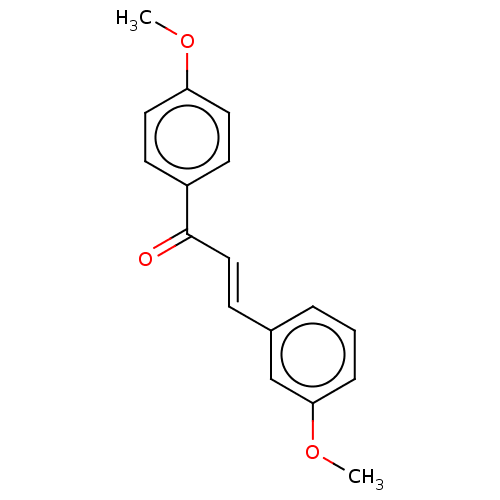 (CHEMBL3613068) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868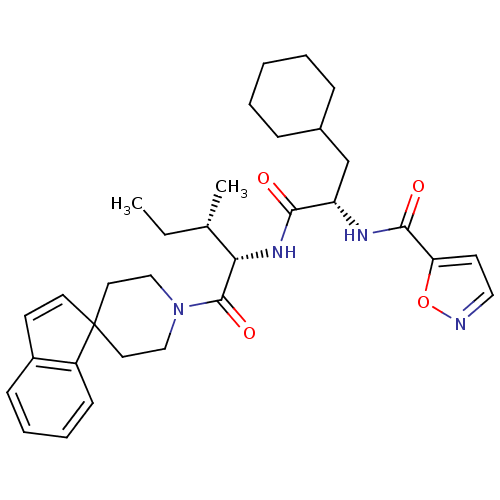 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Antagonist activity at protease-activated receptor 2 (unknown origin) | Bioorg Med Chem 23: 7717-27 (2015) Article DOI: 10.1016/j.bmc.2015.11.016 BindingDB Entry DOI: 10.7270/Q2VH5QP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117980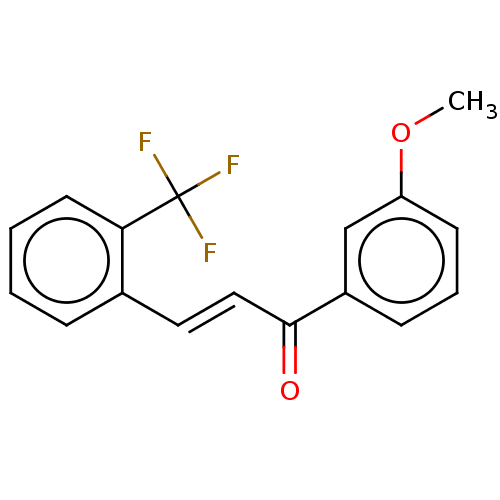 (CHEMBL1801963) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50558208 (CHEMBL4797203) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal His6-tagged human TrkA (440 to end residues) expressed in baculovirus infected Sf21 insect cells | Citation and Details Article DOI: 10.1016/j.bmcl.2016.05.047 BindingDB Entry DOI: 10.7270/Q2CV4NDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118008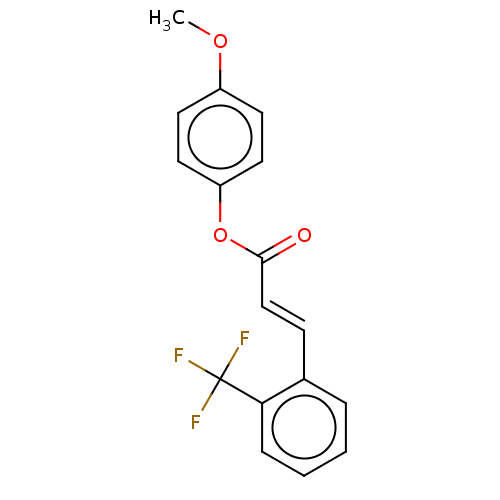 (CHEMBL3613275) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50134197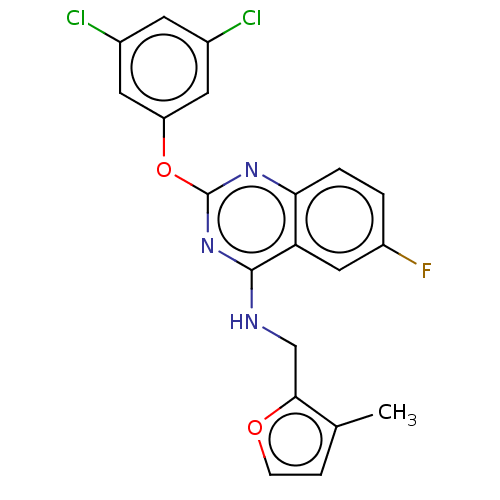 (CHEMBL3735493) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Antagonist activity at protease-activated receptor 2 (unknown origin) expressed in CHO-K1 cells assessed as inhibition of trypsin-induced calcium flu... | Bioorg Med Chem 23: 7717-27 (2015) Article DOI: 10.1016/j.bmc.2015.11.016 BindingDB Entry DOI: 10.7270/Q2VH5QP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM77817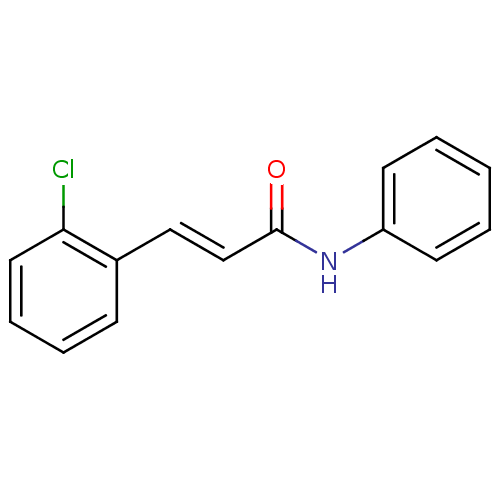 ((E)-3-(2-chlorophenyl)-N-phenyl-2-propenamide | (E...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118002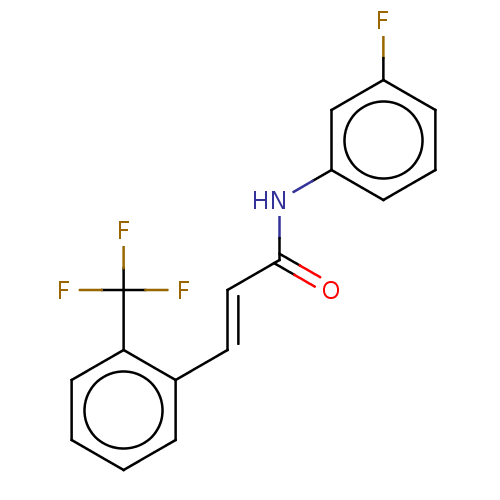 (CHEMBL3613269) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50500968 (CHEMBL3799621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of BRAF V600E mutant (unknown origin) measured after 40 mins by scintillation counting | Eur J Med Chem 115: 201-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.039 BindingDB Entry DOI: 10.7270/Q27947QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118003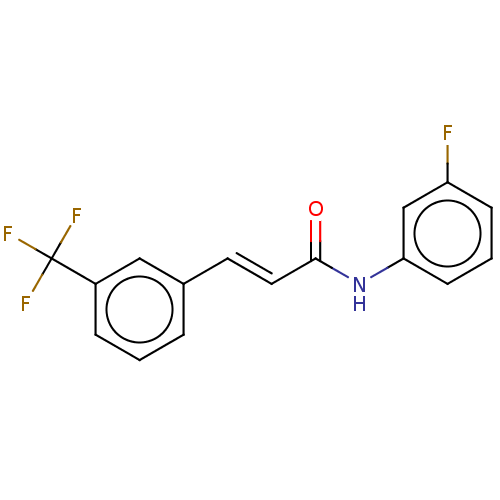 (CHEMBL3613270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM49760 ((E)-3-(2-chlorophenyl)-N-(3-hydroxyphenyl)-2-prope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118007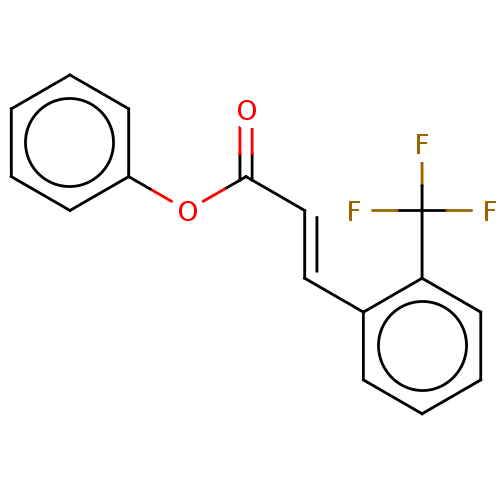 (CHEMBL3613274) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118001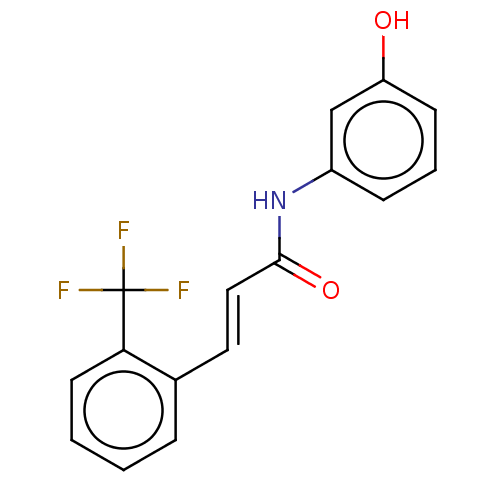 (CHEMBL3613268) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117996 (CHEMBL3613264) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50117999 (CHEMBL3613266) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOA using p-tyramine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50118000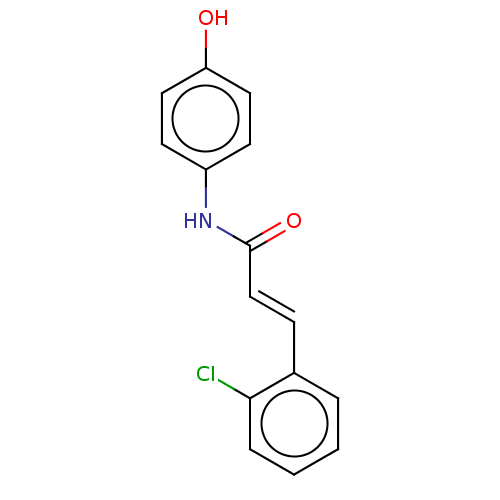 (CHEMBL3613267) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50117999 (CHEMBL3613266) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB using benzylamine as substrate after 30 mins by Amplex red based spectrophotometric analysis | Bioorg Med Chem 23: 6486-96 (2015) Article DOI: 10.1016/j.bmc.2015.08.012 BindingDB Entry DOI: 10.7270/Q2MK6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 105 total ) | Next | Last >> |