 Found 539 hits with Last Name = 'li' and Initial = 'ng'
Found 539 hits with Last Name = 'li' and Initial = 'ng' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50277608

(CHEMBL4173067)Show SMILES COc1ccc(Nc2nccc(n2)-c2c[nH]c3ccccc23)c(OC)c1 Show InChI InChI=1S/C20H18N4O2/c1-25-13-7-8-18(19(11-13)26-2)24-20-21-10-9-17(23-20)15-12-22-16-6-4-3-5-14(15)16/h3-12,22H,1-2H3,(H,21,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50277608

(CHEMBL4173067)Show SMILES COc1ccc(Nc2nccc(n2)-c2c[nH]c3ccccc23)c(OC)c1 Show InChI InChI=1S/C20H18N4O2/c1-25-13-7-8-18(19(11-13)26-2)24-20-21-10-9-17(23-20)15-12-22-16-6-4-3-5-14(15)16/h3-12,22H,1-2H3,(H,21,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275437
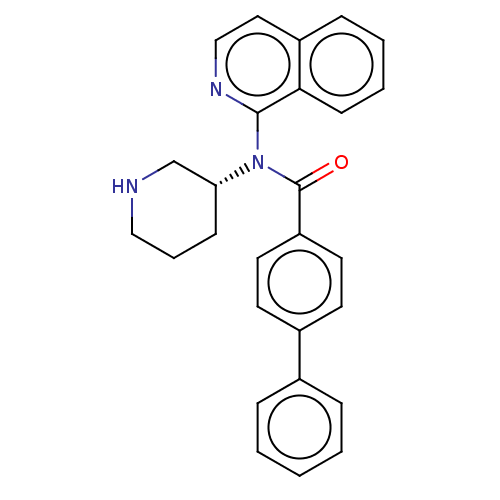
(CHEMBL4129620)Show SMILES O=C(N([C@@H]1CCCNC1)c1nccc2ccccc12)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25N3O/c31-27(23-14-12-21(13-15-23)20-7-2-1-3-8-20)30(24-10-6-17-28-19-24)26-25-11-5-4-9-22(25)16-18-29-26/h1-5,7-9,11-16,18,24,28H,6,10,17,19H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM200318
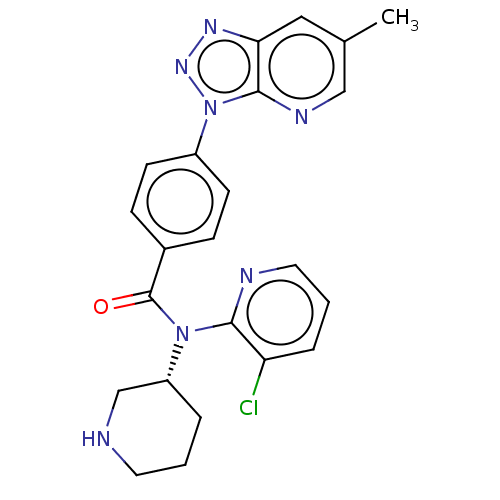
(US9227956, 3)Show SMILES Cc1cnc2n(nnc2c1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C23H22ClN7O/c1-15-12-20-22(27-13-15)31(29-28-20)17-8-6-16(7-9-17)23(32)30(18-4-2-10-25-14-18)21-19(24)5-3-11-26-21/h3,5-9,11-13,18,25H,2,4,10,14H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275430

(CHEMBL4130157)Show SMILES O=C(N([C@@H]1CCCNC1)c1nccc2ccccc12)c1ccc(cc1)-c1cnn2cccnc12 |r| Show InChI InChI=1S/C27H24N6O/c34-27(21-10-8-20(9-11-21)24-18-31-32-16-4-14-29-25(24)32)33(22-6-3-13-28-17-22)26-23-7-2-1-5-19(23)12-15-30-26/h1-2,4-5,7-12,14-16,18,22,28H,3,6,13,17H2/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM200324
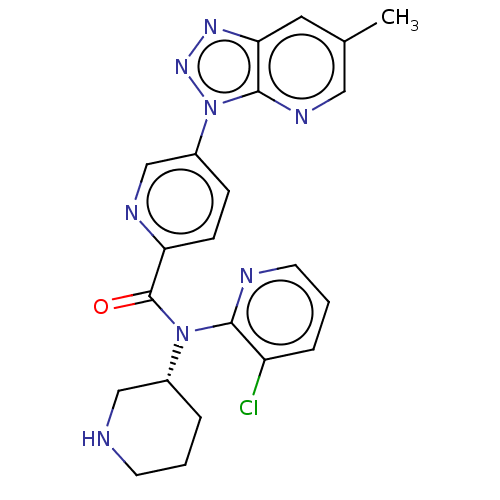
(US9227956, 9)Show SMILES Cc1cnc2n(nnc2c1)-c1ccc(nc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C22H21ClN8O/c1-14-10-19-21(27-11-14)31(29-28-19)16-6-7-18(26-13-16)22(32)30(15-4-2-8-24-12-15)20-17(23)5-3-9-25-20/h3,5-7,9-11,13,15,24H,2,4,8,12H2,1H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275486
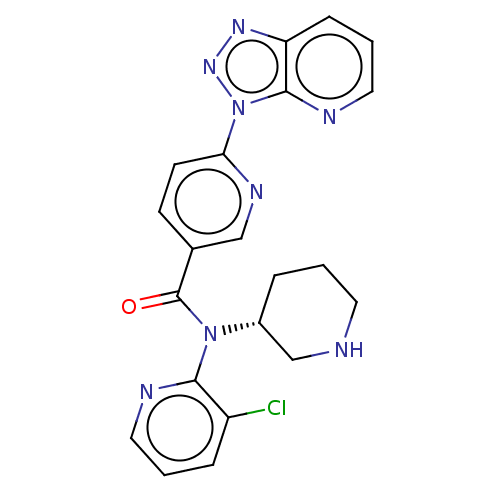
(CHEMBL4128560)Show SMILES Clc1cccnc1N([C@@H]1CCCNC1)C(=O)c1ccc(nc1)-n1nnc2cccnc12 |r| Show InChI InChI=1S/C21H19ClN8O/c22-16-5-2-10-24-19(16)29(15-4-1-9-23-13-15)21(31)14-7-8-18(26-12-14)30-20-17(27-28-30)6-3-11-25-20/h2-3,5-8,10-12,15,23H,1,4,9,13H2/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275436

(CHEMBL4128250)Show SMILES COc1ccc(CCC(=O)N([C@@H]2CCCNC2)c2nccc3ccccc23)cc1 |r| Show InChI InChI=1S/C24H27N3O2/c1-29-21-11-8-18(9-12-21)10-13-23(28)27(20-6-4-15-25-17-20)24-22-7-3-2-5-19(22)14-16-26-24/h2-3,5,7-9,11-12,14,16,20,25H,4,6,10,13,15,17H2,1H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275433
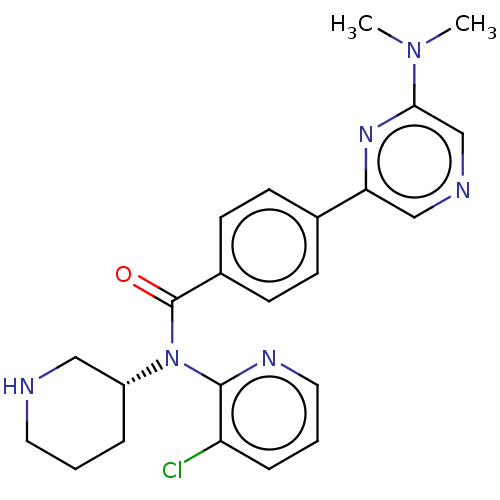
(CHEMBL4127311)Show SMILES CN(C)c1cncc(n1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C23H25ClN6O/c1-29(2)21-15-26-14-20(28-21)16-7-9-17(10-8-16)23(31)30(18-5-3-11-25-13-18)22-19(24)6-4-12-27-22/h4,6-10,12,14-15,18,25H,3,5,11,13H2,1-2H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275485
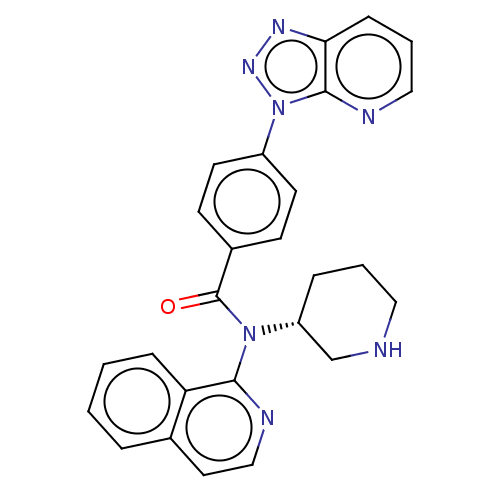
(CHEMBL4126894)Show SMILES O=C(N([C@@H]1CCCNC1)c1nccc2ccccc12)c1ccc(cc1)-n1nnc2cccnc12 |r| Show InChI InChI=1S/C26H23N7O/c34-26(19-9-11-20(12-10-19)33-25-23(30-31-33)8-4-15-28-25)32(21-6-3-14-27-17-21)24-22-7-2-1-5-18(22)13-16-29-24/h1-2,4-5,7-13,15-16,21,27H,3,6,14,17H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275484
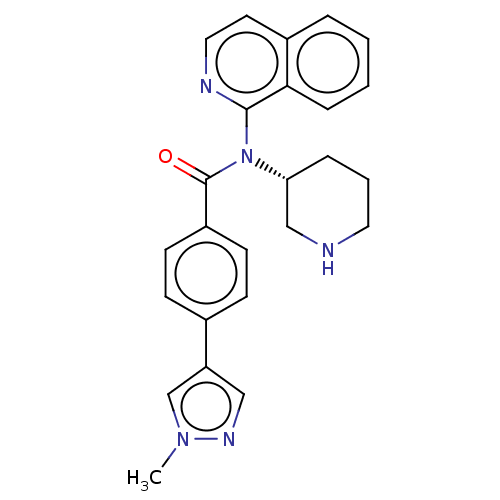
(CHEMBL4128388)Show SMILES Cn1cc(cn1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1nccc2ccccc12 |r| Show InChI InChI=1S/C25H25N5O/c1-29-17-21(15-28-29)18-8-10-20(11-9-18)25(31)30(22-6-4-13-26-16-22)24-23-7-3-2-5-19(23)12-14-27-24/h2-3,5,7-12,14-15,17,22,26H,4,6,13,16H2,1H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM200332
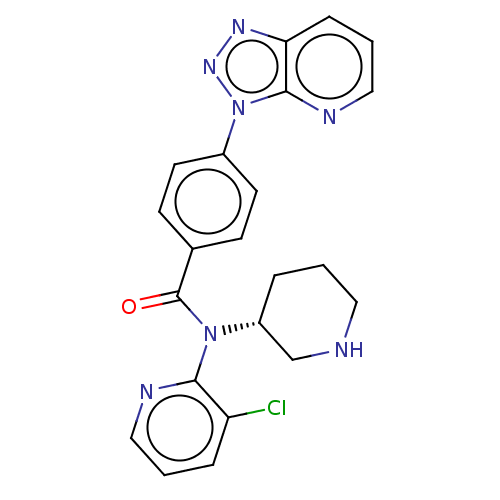
(US9227956, 16)Show SMILES Clc1cccnc1N([C@@H]1CCCNC1)C(=O)c1ccc(cc1)-n1nnc2cccnc12 |r| Show InChI InChI=1S/C22H20ClN7O/c23-18-5-2-12-25-20(18)29(17-4-1-11-24-14-17)22(31)15-7-9-16(10-8-15)30-21-19(27-28-30)6-3-13-26-21/h2-3,5-10,12-13,17,24H,1,4,11,14H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM200320
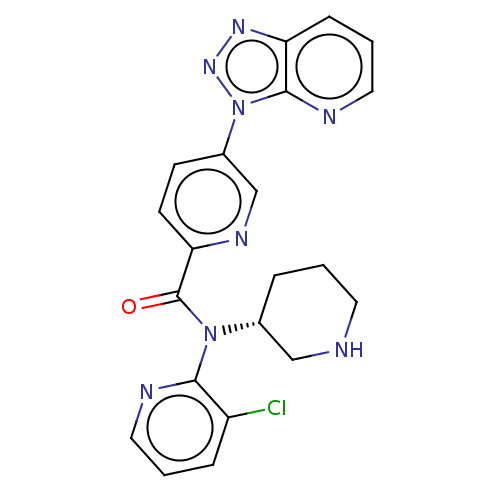
(US9227956, 5)Show SMILES Clc1cccnc1N([C@@H]1CCCNC1)C(=O)c1ccc(cn1)-n1nnc2cccnc12 |r| Show InChI InChI=1S/C21H19ClN8O/c22-16-5-2-10-24-19(16)29(14-4-1-9-23-12-14)21(31)18-8-7-15(13-26-18)30-20-17(27-28-30)6-3-11-25-20/h2-3,5-8,10-11,13-14,23H,1,4,9,12H2/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275487
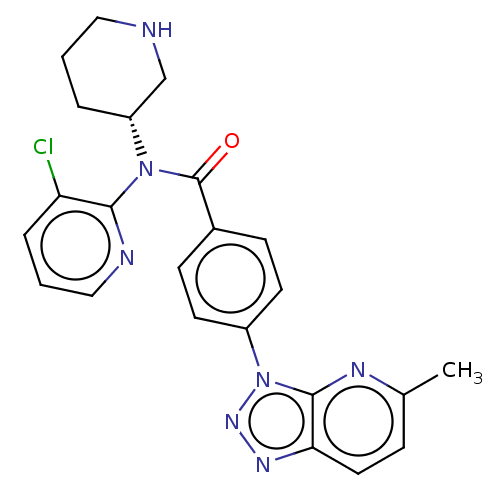
(CHEMBL4126496)Show SMILES Cc1ccc2nnn(-c3ccc(cc3)C(=O)N([C@@H]3CCCNC3)c3ncccc3Cl)c2n1 |r| Show InChI InChI=1S/C23H22ClN7O/c1-15-6-11-20-22(27-15)31(29-28-20)17-9-7-16(8-10-17)23(32)30(18-4-2-12-25-14-18)21-19(24)5-3-13-26-21/h3,5-11,13,18,25H,2,4,12,14H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275434
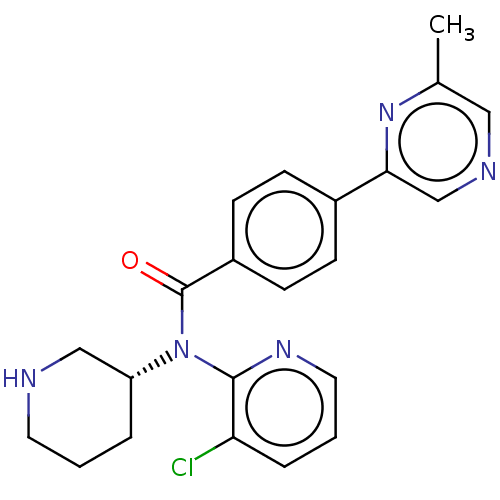
(CHEMBL4127458)Show SMILES Cc1cncc(n1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C22H22ClN5O/c1-15-12-25-14-20(27-15)16-6-8-17(9-7-16)22(29)28(18-4-2-10-24-13-18)21-19(23)5-3-11-26-21/h3,5-9,11-12,14,18,24H,2,4,10,13H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275435
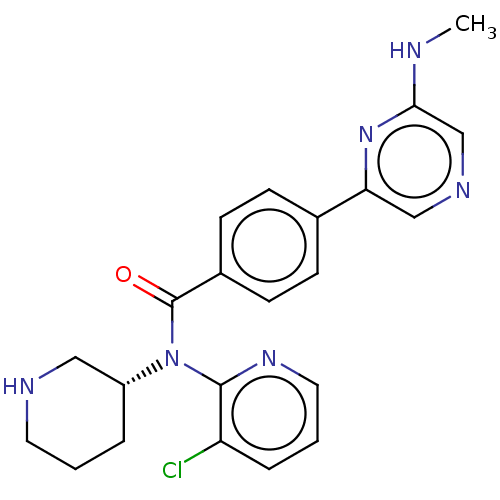
(CHEMBL4126072)Show SMILES CNc1cncc(n1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C22H23ClN6O/c1-24-20-14-26-13-19(28-20)15-6-8-16(9-7-15)22(30)29(17-4-2-10-25-12-17)21-18(23)5-3-11-27-21/h3,5-9,11,13-14,17,25H,2,4,10,12H2,1H3,(H,24,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275431
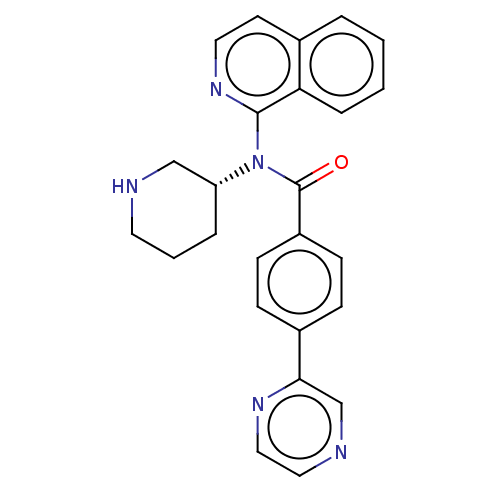
(CHEMBL4127016)Show SMILES O=C(N([C@@H]1CCCNC1)c1nccc2ccccc12)c1ccc(cc1)-c1cnccn1 |r| Show InChI InChI=1S/C25H23N5O/c31-25(20-9-7-19(8-10-20)23-17-27-14-15-28-23)30(21-5-3-12-26-16-21)24-22-6-2-1-4-18(22)11-13-29-24/h1-2,4,6-11,13-15,17,21,26H,3,5,12,16H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149
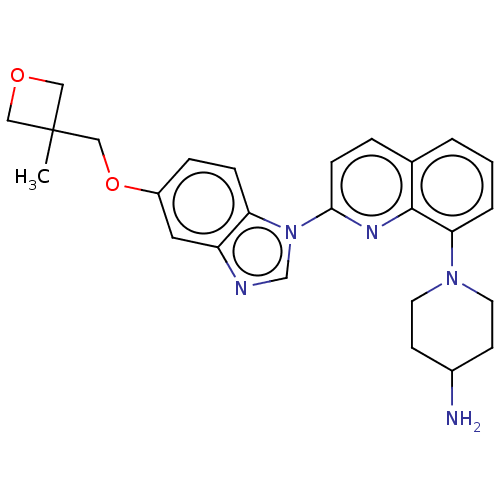
(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM144315
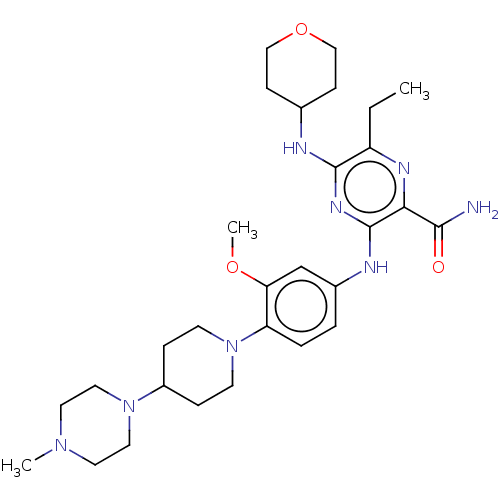
(Gilteritinib | US11512074, Example T-9 | US8969336...)Show SMILES CCc1nc(C(N)=O)c(Nc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1NC1CCOCC1 Show InChI InChI=1S/C29H44N8O3/c1-4-23-28(31-20-9-17-40-18-10-20)34-29(26(33-23)27(30)38)32-21-5-6-24(25(19-21)39-3)37-11-7-22(8-12-37)36-15-13-35(2)14-16-36/h5-6,19-20,22H,4,7-18H2,1-3H3,(H2,30,38)(H2,31,32,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255355
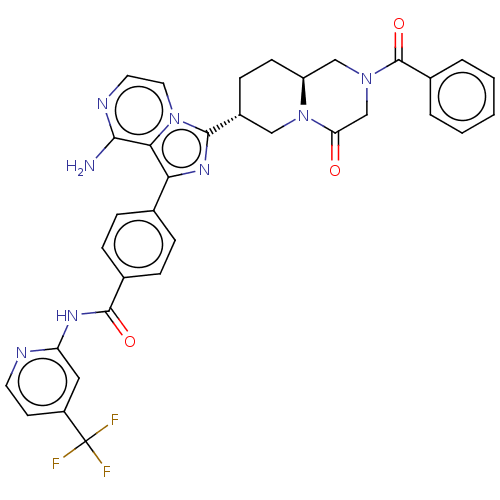
(US9481682, 105)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CN(CC(=O)N2C1)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C34H29F3N8O3/c35-34(36,37)24-12-13-39-26(16-24)41-32(47)21-8-6-20(7-9-21)28-29-30(38)40-14-15-44(29)31(42-28)23-10-11-25-18-43(19-27(46)45(25)17-23)33(48)22-4-2-1-3-5-22/h1-9,12-16,23,25H,10-11,17-19H2,(H2,38,40)(H,39,41,47)/t23-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255335

(US9481682, 85)Show SMILES COc1cc(ccc1-c1nc([C@@H]2CC[C@H]3CN(CC(=O)N3C2)C(=O)c2ccncc2)n2ccnc(N)c12)C(=O)Nc1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C34H30F3N9O4/c1-50-25-14-20(32(48)42-26-15-22(8-11-40-26)34(35,36)37)3-5-24(25)28-29-30(38)41-12-13-45(29)31(43-28)21-2-4-23-17-44(18-27(47)46(23)16-21)33(49)19-6-9-39-10-7-19/h3,5-15,21,23H,2,4,16-18H2,1H3,(H2,38,41)(H,40,42,48)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM144315

(Gilteritinib | US11512074, Example T-9 | US8969336...)Show SMILES CCc1nc(C(N)=O)c(Nc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1NC1CCOCC1 Show InChI InChI=1S/C29H44N8O3/c1-4-23-28(31-20-9-17-40-18-10-20)34-29(26(33-23)27(30)38)32-21-5-6-24(25(19-21)39-3)37-11-7-22(8-12-37)36-15-13-35(2)14-16-36/h5-6,19-20,22H,4,7-18H2,1-3H3,(H2,30,38)(H2,31,32,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM13535
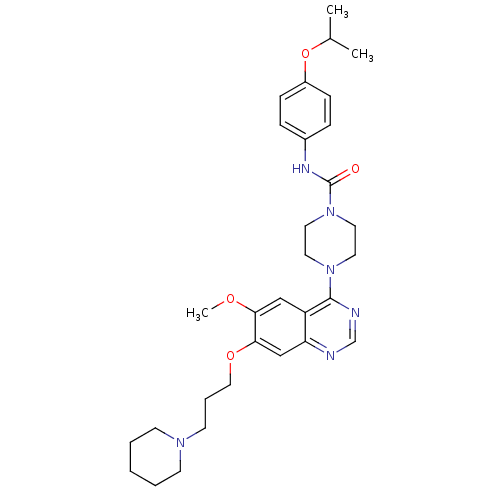
(4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin...)Show SMILES COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-23(2)41-25-10-8-24(9-11-25)34-31(38)37-17-15-36(16-18-37)30-26-20-28(39-3)29(21-27(26)32-22-33-30)40-19-7-14-35-12-5-4-6-13-35/h8-11,20-23H,4-7,12-19H2,1-3H3,(H,34,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149

(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149

(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594234

(CHEMBL5200336)Show SMILES Cc1cc(C(N)=O)c2[nH]c3cc(ccc3c2c1-c1cccc(c1O)-n1c(=O)[nH]c2c(F)cccc2c1=O)C(C)(C)O |(3.12,1.54,;1.79,2.31,;1.79,3.85,;.47,4.61,;.47,6.15,;1.8,6.92,;-.87,6.92,;-.86,3.85,;-2.33,4.32,;-3.23,3.08,;-4.77,2.91,;-5.39,1.5,;-4.49,.27,;-2.95,.43,;-2.33,1.83,;-.86,2.31,;.47,1.55,;.47,.01,;-.87,-.77,;-.86,-2.31,;.47,-3.07,;1.8,-2.3,;1.8,-.76,;3.14,.01,;3.13,-3.07,;3.13,-4.61,;1.8,-5.38,;4.47,-5.38,;5.8,-4.61,;7.14,-5.38,;7.14,-6.92,;8.47,-4.6,;8.47,-3.07,;7.13,-2.3,;5.8,-3.07,;4.47,-2.3,;4.47,-.76,;-6.93,1.5,;-7.7,.17,;-7.7,2.84,;-8.47,1.5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594232

(CHEMBL5171706)Show SMILES OC[C@H]1CCC[C@H](Nc2ncnc3[nH]cc(C(=O)c4ccc(Oc5ccccc5)cc4Cl)c23)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50422751
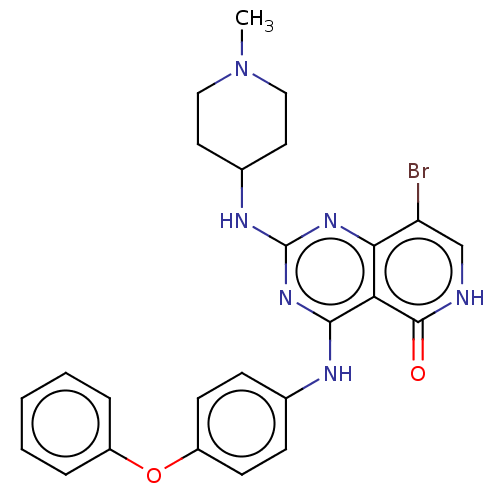
(DENFIVONTINIB | Denfivontinib | G-749)Show SMILES CCCCCCCCN1C(=O)NC(C1=O)(c1ccc(C)cc1)c1ccc(C)cc1 Show InChI InChI=1S/C25H32N2O2/c1-4-5-6-7-8-9-18-27-23(28)25(26-24(27)29,21-14-10-19(2)11-15-21)22-16-12-20(3)13-17-22/h10-17H,4-9,18H2,1-3H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50056199
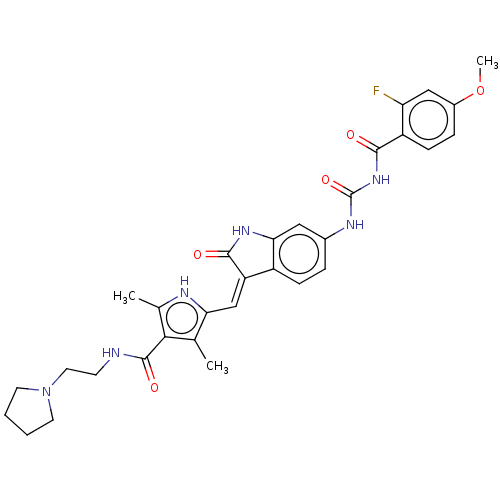
(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50422749
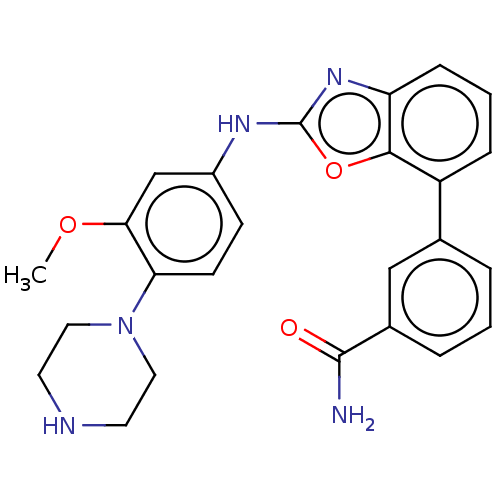
(CHEMBL5287891)Show SMILES CCCCN1C(=S)NC(C1=O)(c1ccc(Cl)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H18Cl2N2OS/c1-2-3-12-23-17(24)19(22-18(23)25,13-4-8-15(20)9-5-13)14-6-10-16(21)11-7-14/h4-11H,2-3,12H2,1H3,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50422752
(CHEMBL5284731)Show InChI InChI=1S/C22H26N2OS/c1-2-3-4-5-12-17-24-20(25)22(23-21(24)26,18-13-8-6-9-14-18)19-15-10-7-11-16-19/h6-11,13-16H,2-5,12,17H2,1H3,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
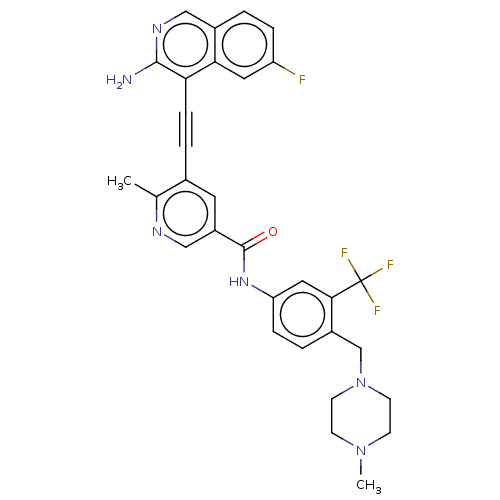
(Homo sapiens (Human)) | BDBM50608318
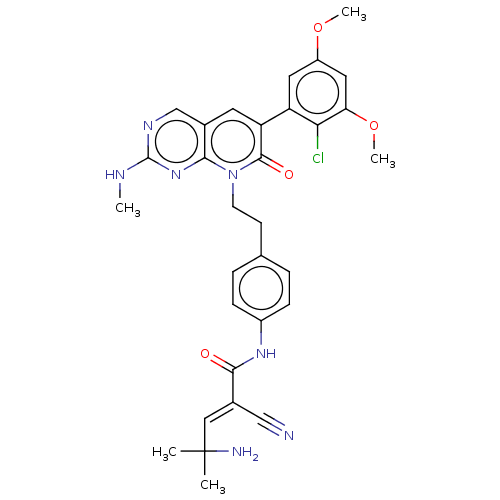
(CHEMBL5279959)Show SMILES CNc1ncc2cc(-c3cc(OC)cc(OC)c3Cl)c(=O)n(CCc3ccc(NC(=O)C(=C\C(C)(C)N)\C#N)cc3)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50380950

(CHEMBL2016905)Show SMILES Fc1ccc2[nH]c(=O)c(cc2c1)-c1nc2CCN(Cc2[nH]1)C(=O)c1ccc(CN2CCCCC2)cc1 Show InChI InChI=1S/C28H28FN5O2/c29-21-8-9-23-20(14-21)15-22(27(35)32-23)26-30-24-10-13-34(17-25(24)31-26)28(36)19-6-4-18(5-7-19)16-33-11-2-1-3-12-33/h4-9,14-15H,1-3,10-13,16-17H2,(H,30,31)(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50422751

(DENFIVONTINIB | Denfivontinib | G-749)Show SMILES CCCCCCCCN1C(=O)NC(C1=O)(c1ccc(C)cc1)c1ccc(C)cc1 Show InChI InChI=1S/C25H32N2O2/c1-4-5-6-7-8-9-18-27-23(28)25(26-24(27)29,21-14-10-19(2)11-15-21)22-16-12-20(3)13-17-22/h10-17H,4-9,18H2,1-3H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM3033
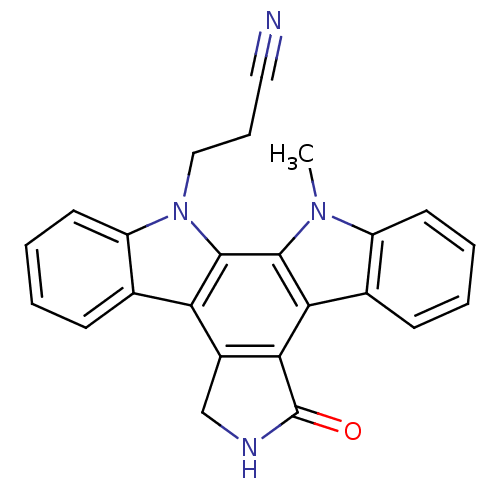
(3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0...)Show SMILES Cn1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4n(CCC#N)c3c12 Show InChI InChI=1S/C24H18N4O/c1-27-17-9-4-2-7-14(17)20-21-16(13-26-24(21)29)19-15-8-3-5-10-18(15)28(12-6-11-25)23(19)22(20)27/h2-5,7-10H,6,12-13H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50055496
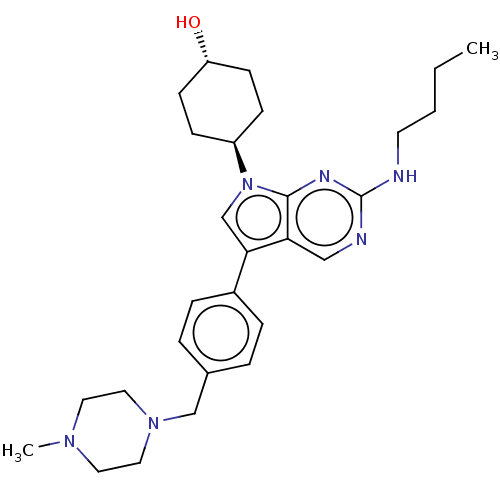
(CHEMBL3326006)Show SMILES CCCCNc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)-c1ccc(CN2CCN(C)CC2)cc1 |r,wU:12.11,wD:15.15,(14.5,-10.38,;14.49,-11.92,;15.83,-12.69,;17.16,-11.92,;18.49,-12.69,;19.83,-11.92,;19.83,-10.38,;21.16,-9.61,;22.49,-10.37,;23.97,-9.89,;24.88,-11.15,;23.97,-12.4,;24.44,-13.87,;25.95,-14.19,;26.42,-15.66,;25.39,-16.8,;25.86,-18.27,;23.88,-16.47,;23.41,-15.02,;22.49,-11.92,;21.16,-12.69,;24.45,-8.43,;25.96,-8.11,;26.43,-6.65,;25.41,-5.5,;25.89,-4.03,;27.4,-3.74,;28.41,-4.9,;29.91,-4.6,;30.41,-3.14,;31.92,-2.84,;29.4,-1.99,;27.88,-2.28,;23.9,-5.82,;23.42,-7.29,)| Show InChI InChI=1S/C28H40N6O/c1-3-4-13-29-28-30-18-25-26(20-34(27(25)31-28)23-9-11-24(35)12-10-23)22-7-5-21(6-8-22)19-33-16-14-32(2)15-17-33/h5-8,18,20,23-24,35H,3-4,9-17,19H2,1-2H3,(H,29,30,31)/t23-,24- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594232

(CHEMBL5171706)Show SMILES OC[C@H]1CCC[C@H](Nc2ncnc3[nH]cc(C(=O)c4ccc(Oc5ccccc5)cc4Cl)c23)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50326053
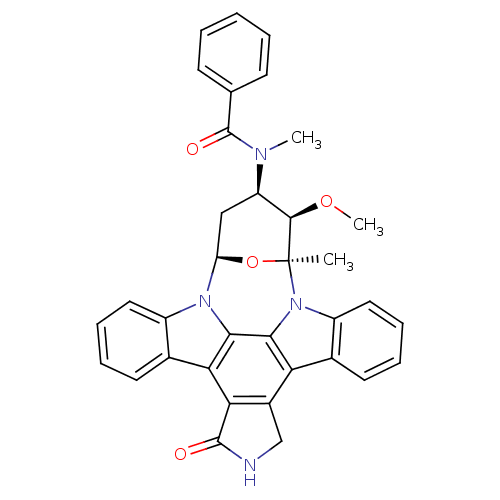
(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50185178

(1-tert-butyl-3-(3-(5-(4-(piperidin-1-yl)piperidin-...)Show SMILES CC(C)(C)NC(=O)Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1 Show InChI InChI=1S/C29H38N8O/c1-29(2,3)33-28(38)30-19-7-9-23-22(17-19)26(35-34-23)27-31-24-10-8-21(18-25(24)32-27)37-15-11-20(12-16-37)36-13-5-4-6-14-36/h7-10,17-18,20H,4-6,11-16H2,1-3H3,(H,31,32)(H,34,35)(H2,30,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50601438
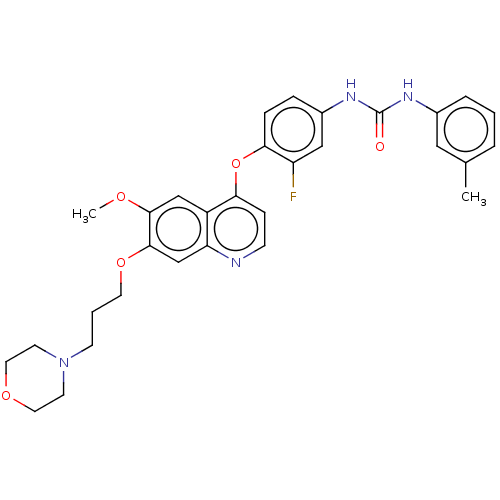
(CHEMBL5170277)Show SMILES COc1cc2c(Oc3ccc(NC(=O)Nc4cccc(C)c4)cc3F)ccnc2cc1OCCCN1CCOCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594233
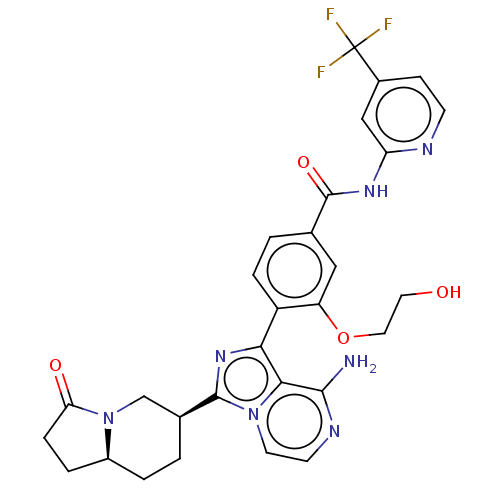
(CHEMBL5175002)Show SMILES [H][C@]12CCC(=O)N1C[C@H](CC2)c1nc(-c2ccc(cc2OCCO)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418177
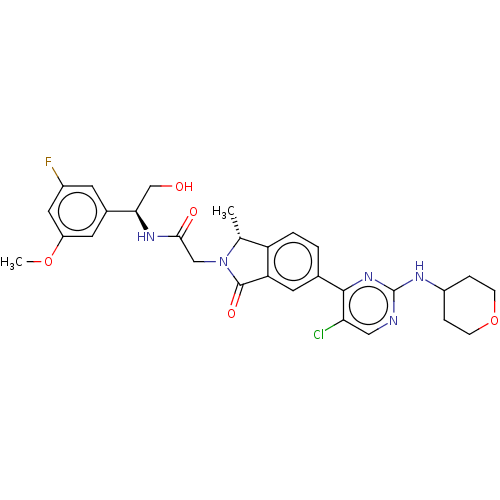
(2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...)Show SMILES COc1cc(F)cc(c1)[C@@H](CO)NC(=O)CN1[C@H](C)c2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C29H31ClFN5O5/c1-16-22-4-3-17(27-24(30)13-32-29(35-27)33-20-5-7-41-8-6-20)11-23(22)28(39)36(16)14-26(38)34-25(15-37)18-9-19(31)12-21(10-18)40-2/h3-4,9-13,16,20,25,37H,5-8,14-15H2,1-2H3,(H,34,38)(H,32,33,35)/t16-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00905
BindingDB Entry DOI: 10.7270/Q2BP06M5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50573875

(CHEMBL4869086)Show SMILES COc1cc(F)cc(c1)[C@@H](CO)NC(=O)CN1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00905
BindingDB Entry DOI: 10.7270/Q2BP06M5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM417999
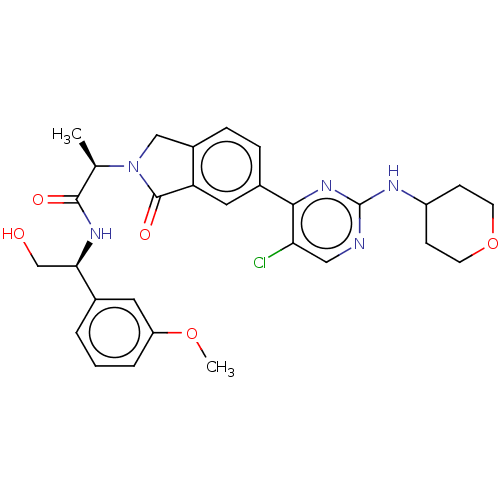
(US10457669, Example 675 | US11001575, Example 675)Show SMILES COc1cccc(c1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C29H32ClN5O5/c1-17(27(37)33-25(16-36)18-4-3-5-22(12-18)39-2)35-15-20-7-6-19(13-23(20)28(35)38)26-24(30)14-31-29(34-26)32-21-8-10-40-11-9-21/h3-7,12-14,17,21,25,36H,8-11,15-16H2,1-2H3,(H,33,37)(H,31,32,34)/t17-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00905
BindingDB Entry DOI: 10.7270/Q2BP06M5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255370

(US9481682, 121)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3OC3CC3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CCC(=O)N2C1 |r| Show InChI InChI=1S/C30H28F3N7O3/c31-30(32,33)18-9-10-35-23(14-18)37-29(42)16-2-7-21(22(13-16)43-20-5-6-20)25-26-27(34)36-11-12-39(26)28(38-25)17-1-3-19-4-8-24(41)40(19)15-17/h2,7,9-14,17,19-20H,1,3-6,8,15H2,(H2,34,36)(H,35,37,42)/t17-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50573874
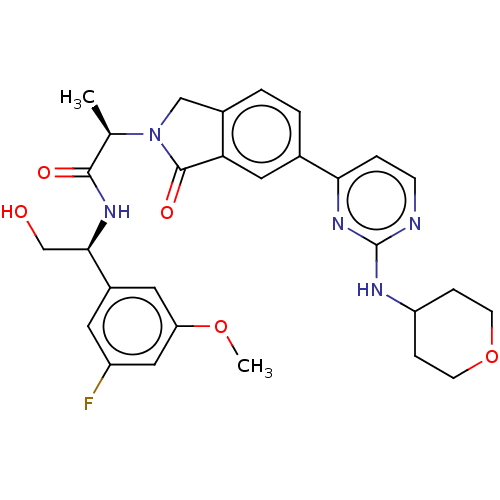
(CHEMBL4873851)Show SMILES COc1cc(F)cc(c1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00905
BindingDB Entry DOI: 10.7270/Q2BP06M5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418019

(US10457669, Example 698 | US11001575, Example 698)Show SMILES COc1cc(ccn1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C28H31ClN6O5/c1-16(26(37)33-23(15-36)17-5-8-30-24(12-17)39-2)35-14-19-4-3-18(11-21(19)27(35)38)25-22(29)13-31-28(34-25)32-20-6-9-40-10-7-20/h3-5,8,11-13,16,20,23,36H,6-7,9-10,14-15H2,1-2H3,(H,33,37)(H,31,32,34)/t16-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00905
BindingDB Entry DOI: 10.7270/Q2BP06M5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data