

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as inhibition constant using acetylthiocholine iodide as substrate by Cornish-Bowden plot an... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as dissociation constant for protein-substrate-compound complex using acetylthiocholine iodi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456669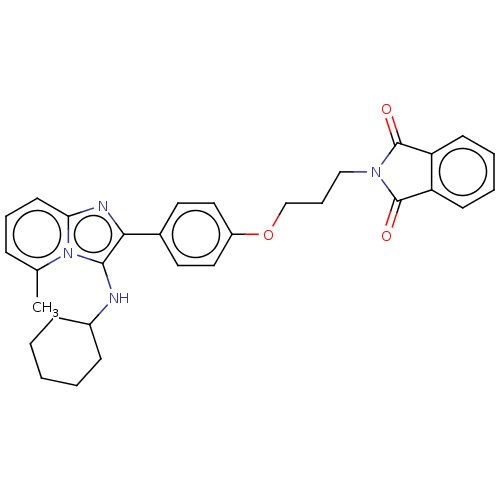 (CHEMBL4203158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456675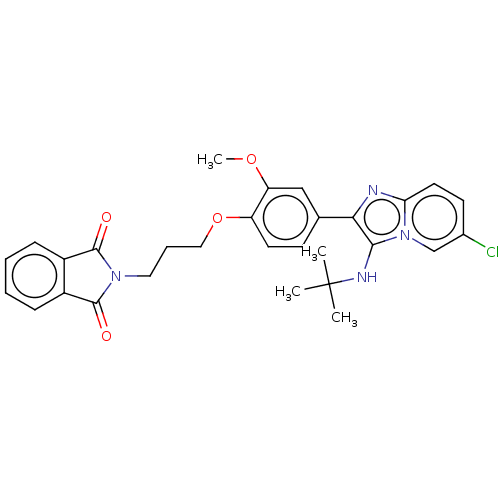 (CHEMBL4212743) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456672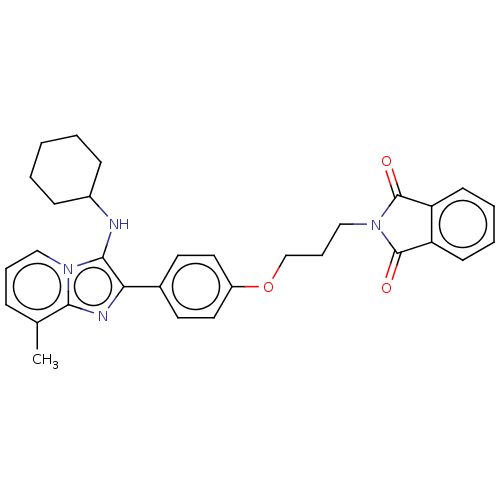 (CHEMBL4211862) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456670 (CHEMBL4210406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456677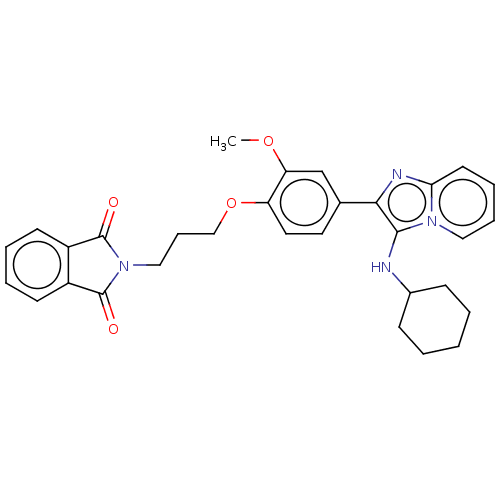 (CHEMBL4214810) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456671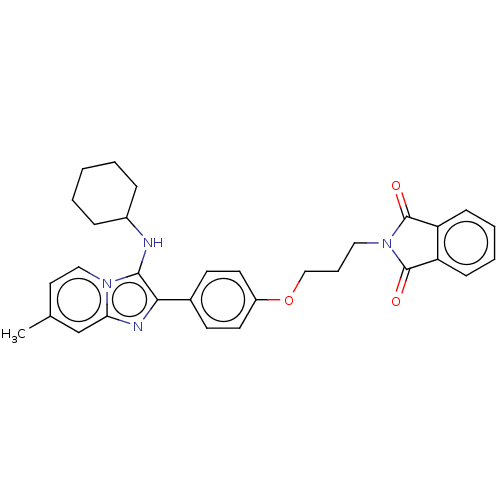 (CHEMBL4215348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456678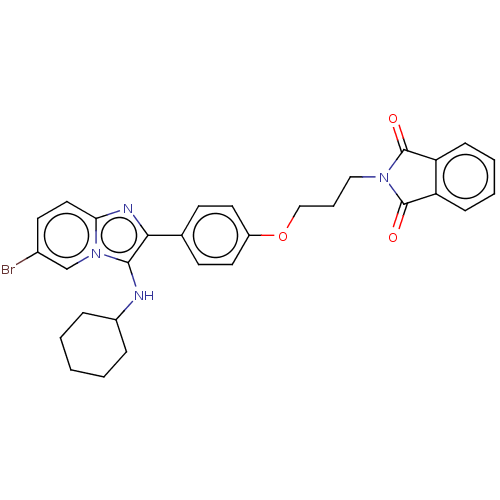 (CHEMBL4205452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456673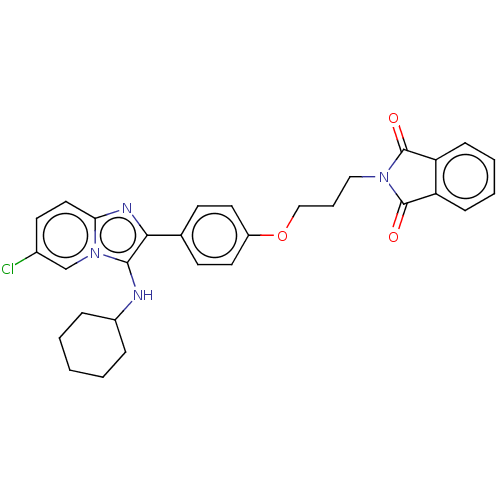 (CHEMBL4213285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456680 (CHEMBL4208354) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456676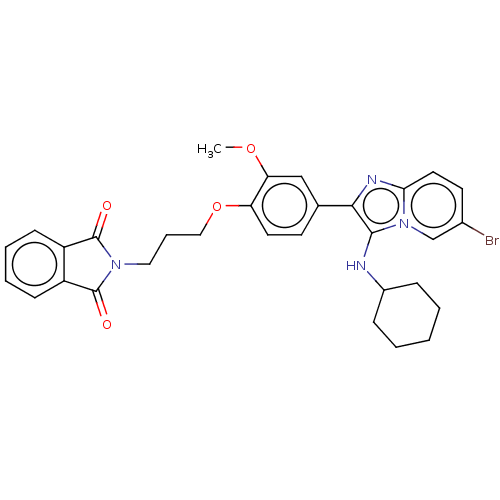 (CHEMBL4217172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456668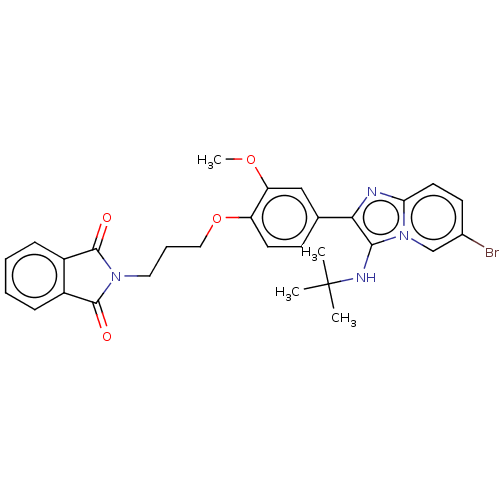 (CHEMBL4204959) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456679 (CHEMBL4206975) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456674 (CHEMBL4217695) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456667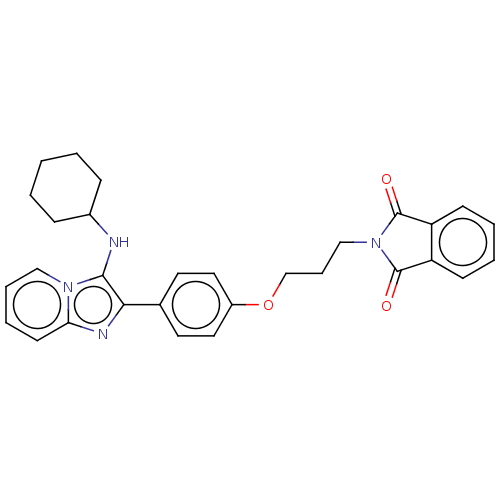 (CHEMBL4206562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50456681 (CHEMBL4209831) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM47353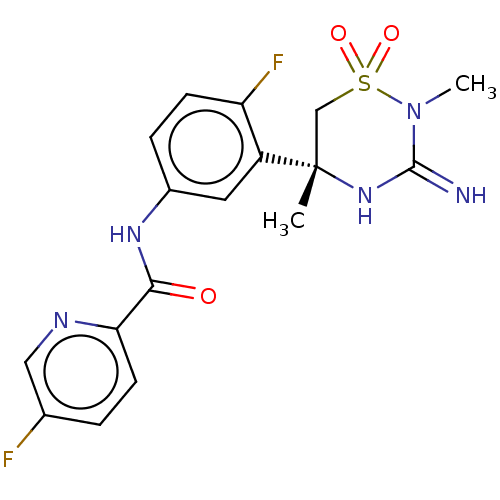 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556958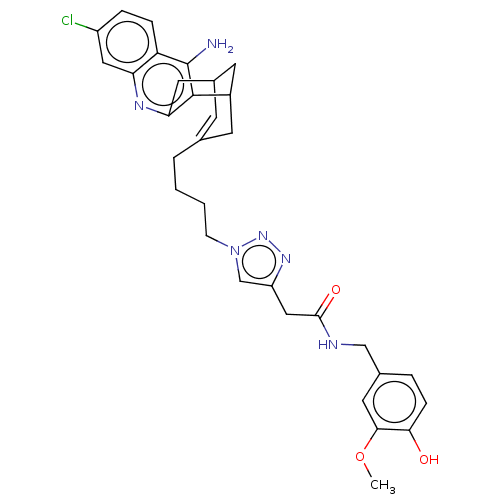 (CHEMBL4744528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556953 (CHEMBL4748114) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556954 (CHEMBL4782921) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50494043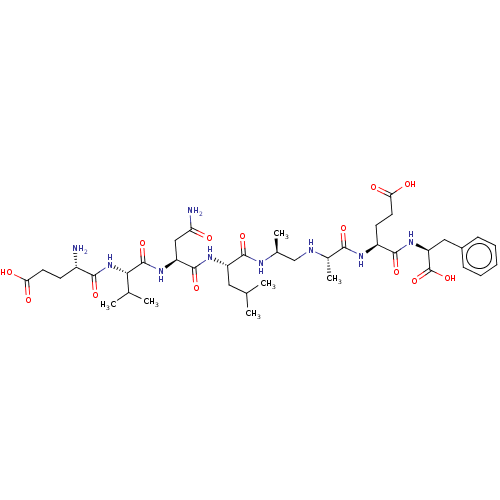 (CHEMBL2443369) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shiraz University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE-1 using SEVNLDAEFK as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 6893-909 (2013) Article DOI: 10.1016/j.bmc.2013.09.033 BindingDB Entry DOI: 10.7270/Q2HQ42V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556956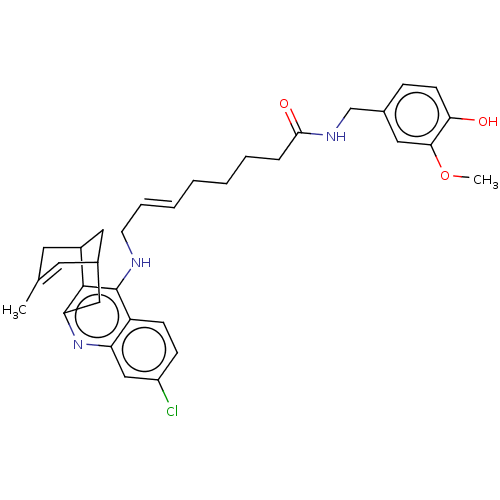 (CHEMBL4741162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556951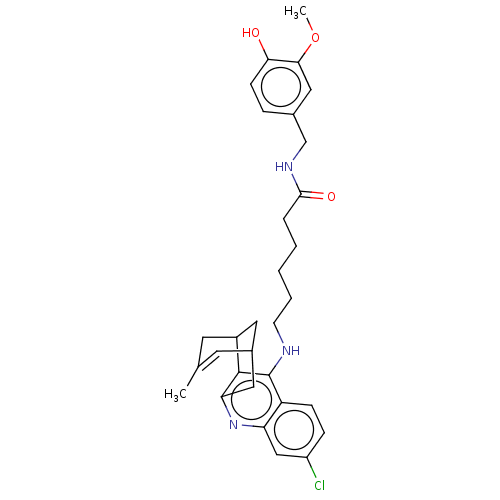 (CHEMBL4788434) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556950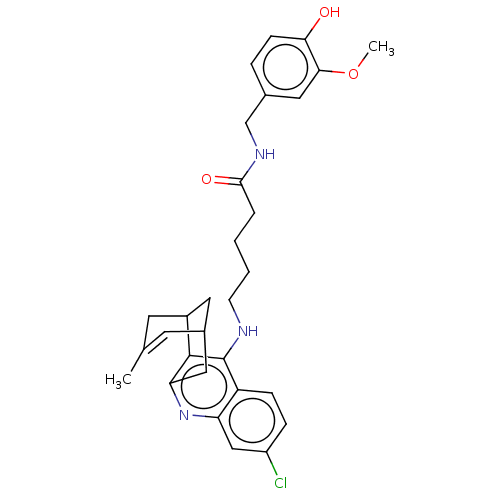 (CHEMBL4743210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556957 (CHEMBL4794769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556958 (CHEMBL4744528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tehran Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in baculovirus expression system using Rh-EVNLDAEFK-quencher as substrate after 90 mins by FR... | Eur J Med Chem 138: 729-737 (2017) Article DOI: 10.1016/j.ejmech.2017.06.040 BindingDB Entry DOI: 10.7270/Q2NC63S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Shiraz University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 (1 to 460 residues) expressed in baculovirus infected insect cells using Rh-EVNLDAEFK-Quencher as substrate inc... | Eur J Med Chem 141: 690-702 (2017) Article DOI: 10.1016/j.ejmech.2017.09.057 BindingDB Entry DOI: 10.7270/Q2DJ5J45 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556955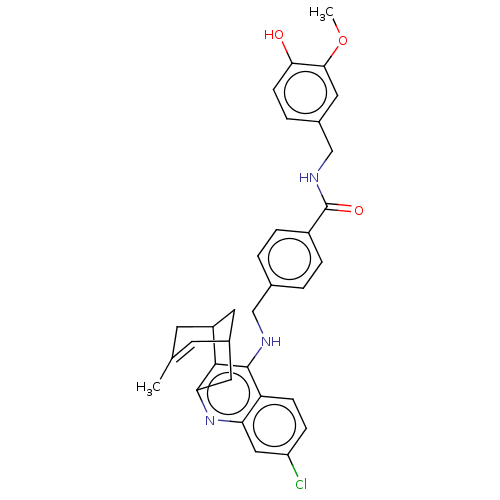 (CHEMBL4782267) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556954 (CHEMBL4782921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556953 (CHEMBL4748114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556950 (CHEMBL4743210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556957 (CHEMBL4794769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556951 (CHEMBL4788434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556956 (CHEMBL4741162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432316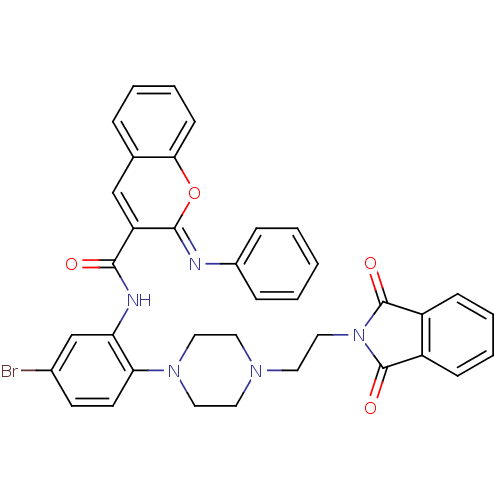 (CHEMBL2347874) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432318 (CHEMBL2347872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556955 (CHEMBL4782267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432315 (CHEMBL2347875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432324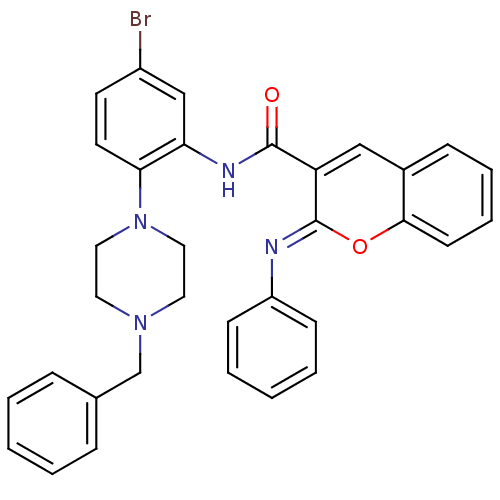 (CHEMBL2347867) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 503 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432317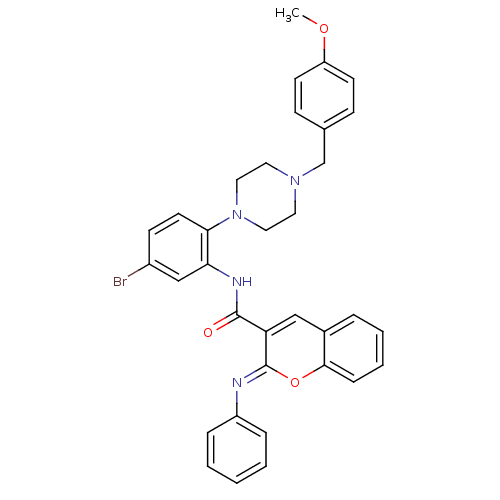 (CHEMBL2347873) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 578 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432308 (CHEMBL2347882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432320 (CHEMBL2347870) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 849 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432314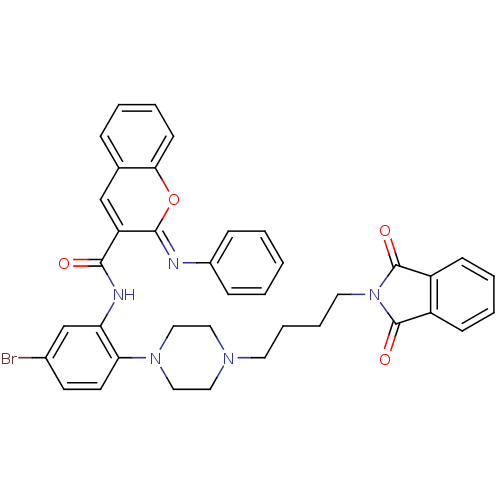 (CHEMBL2347876) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 854 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |