 Found 35 hits with Last Name = 'nyanguile' and Initial = 'o'
Found 35 hits with Last Name = 'nyanguile' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101334
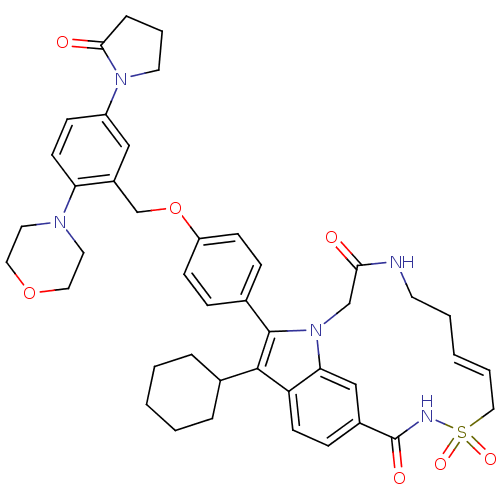
(US8524716, 13)Show SMILES O=C1CCCN1c1ccc(N2CCOCC2)c(COc2ccc(cc2)-c2c(C3CCCCC3)c3ccc4cc3n2CC(=O)NCC\C=C/CS(=O)(=O)NC4=O)c1 |c:52| Show InChI InChI=1S/C43H49N5O7S/c49-39-28-48-38-27-32(43(51)45-56(52,53)25-6-2-5-19-44-39)13-17-36(38)41(30-8-3-1-4-9-30)42(48)31-11-15-35(16-12-31)55-29-33-26-34(47-20-7-10-40(47)50)14-18-37(33)46-21-23-54-24-22-46/h2,6,11-18,26-27,30H,1,3-5,7-10,19-25,28-29H2,(H,44,49)(H,45,51)/b6-2- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101339
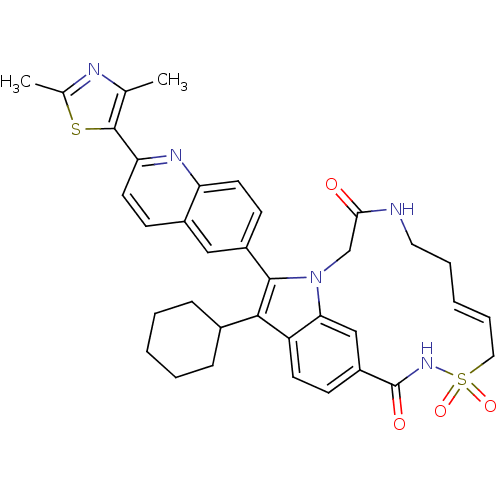
(US8524716, 18)Show SMILES Cc1nc(C)c(s1)-c1ccc2cc(ccc2n1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCC\C=C/CS(=O)(=O)NC3=O |c:44| Show InChI InChI=1S/C36H37N5O4S2/c1-22-35(46-23(2)38-22)30-16-12-25-19-26(13-15-29(25)39-30)34-33(24-9-5-3-6-10-24)28-14-11-27-20-31(28)41(34)21-32(42)37-17-7-4-8-18-47(44,45)40-36(27)43/h4,8,11-16,19-20,24H,3,5-7,9-10,17-18,21H2,1-2H3,(H,37,42)(H,40,43)/b8-4- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101312
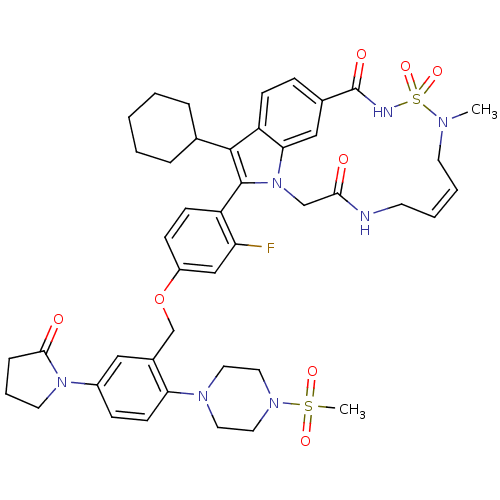
(US8524716, 19)Show SMILES CN1C\C=C/CNC(=O)Cn2c(c(C3CCCCC3)c3ccc(cc23)C(=O)NS1(=O)=O)-c1ccc(OCc2cc(ccc2N2CCN(CC2)S(C)(=O)=O)N2CCCC2=O)cc1F |c:3,(-11.97,-2.04,;-10.64,-1.27,;-9.3,-2.04,;-8.42,-3.82,;-7.08,-4.59,;-3.88,-4.59,;-2.79,-3.5,;-3.19,-2.01,;-4.68,-1.61,;-2.1,-.92,;-2.5,.57,;-1.6,1.81,;-2.5,3.06,;-2.1,4.55,;-.62,4.95,;-.22,6.43,;-1.31,7.52,;-2.79,7.12,;-3.19,5.64,;-3.97,2.58,;-5.3,3.35,;-6.63,2.58,;-6.63,1.04,;-5.3,.27,;-3.97,1.04,;-7.97,.27,;-7.97,-1.27,;-9.3,1.04,;-10.64,.27,;-11.97,1.04,;-10.64,1.81,;-.06,1.81,;.71,3.15,;2.25,3.15,;3.02,1.81,;4.56,1.81,;5.33,.48,;6.87,.48,;7.64,1.81,;9.18,1.81,;9.95,.48,;9.18,-.85,;7.64,-.85,;6.87,-2.19,;5.33,-2.19,;4.56,-3.52,;5.33,-4.85,;6.87,-4.85,;7.64,-3.52,;4.56,-6.19,;5.33,-7.52,;3.02,-6.19,;3.79,-4.85,;9.95,3.15,;11.49,3.15,;11.97,4.61,;10.72,5.52,;9.48,4.61,;8.14,5.38,;2.25,.48,;.71,.48,;-.06,-.85,)| Show InChI InChI=1S/C44H52FN7O8S2/c1-48-19-7-6-18-46-40(53)28-52-39-26-31(44(55)47-62(48,58)59)12-15-36(39)42(30-9-4-3-5-10-30)43(52)35-16-14-34(27-37(35)45)60-29-32-25-33(51-20-8-11-41(51)54)13-17-38(32)49-21-23-50(24-22-49)61(2,56)57/h6-7,12-17,25-27,30H,3-5,8-11,18-24,28-29H2,1-2H3,(H,46,53)(H,47,55)/b7-6- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101322
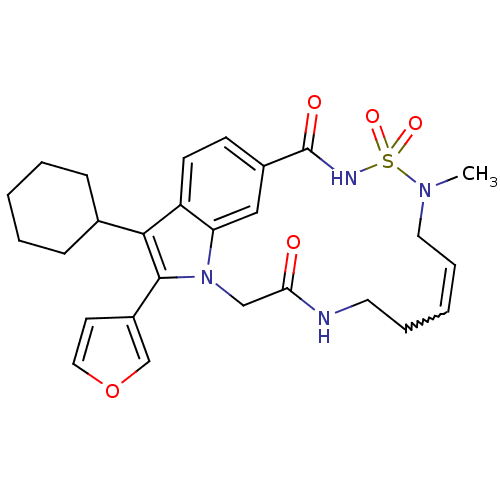
(US8524716, 29)Show SMILES CN1CC=CCCNC(=O)Cn2c(-c3ccoc3)c(C3CCCCC3)c3ccc(cc23)C(=O)NS1(=O)=O |w:4.4| Show InChI InChI=1S/C27H32N4O5S/c1-30-14-7-3-6-13-28-24(32)17-31-23-16-20(27(33)29-37(30,34)35)10-11-22(23)25(19-8-4-2-5-9-19)26(31)21-12-15-36-18-21/h3,7,10-12,15-16,18-19H,2,4-6,8-9,13-14,17H2,1H3,(H,28,32)(H,29,33) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101319
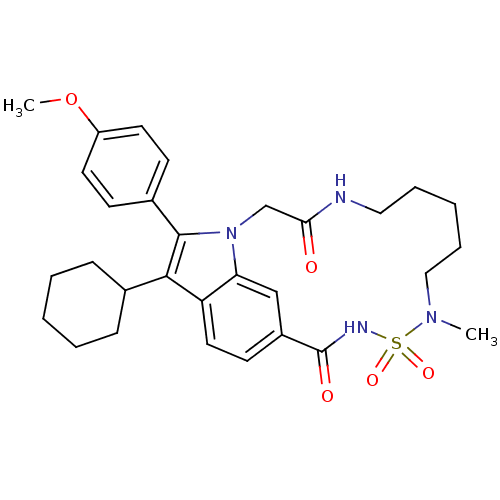
(US8524716, 210 | US8524716, 26)Show SMILES COc1ccc(cc1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCCCCCN(C)S(=O)(=O)NC3=O Show InChI InChI=1S/C30H38N4O5S/c1-33-18-8-4-7-17-31-27(35)20-34-26-19-23(30(36)32-40(33,37)38)13-16-25(26)28(21-9-5-3-6-10-21)29(34)22-11-14-24(39-2)15-12-22/h11-16,19,21H,3-10,17-18,20H2,1-2H3,(H,31,35)(H,32,36) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101323
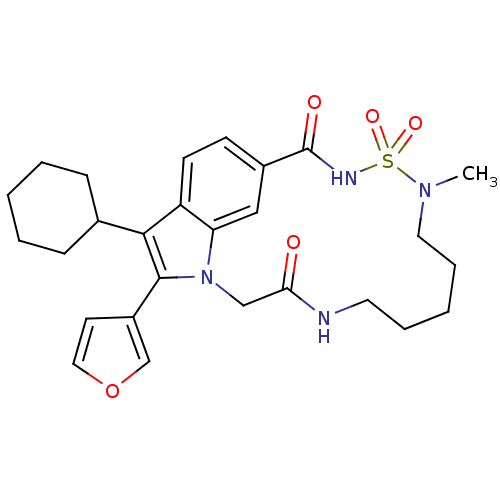
(US8524716, 30)Show SMILES CN1CCCCCNC(=O)Cn2c(-c3ccoc3)c(C3CCCCC3)c3ccc(cc23)C(=O)NS1(=O)=O Show InChI InChI=1S/C27H34N4O5S/c1-30-14-7-3-6-13-28-24(32)17-31-23-16-20(27(33)29-37(30,34)35)10-11-22(23)25(19-8-4-2-5-9-19)26(31)21-12-15-36-18-21/h10-12,15-16,18-19H,2-9,13-14,17H2,1H3,(H,28,32)(H,29,33) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101313
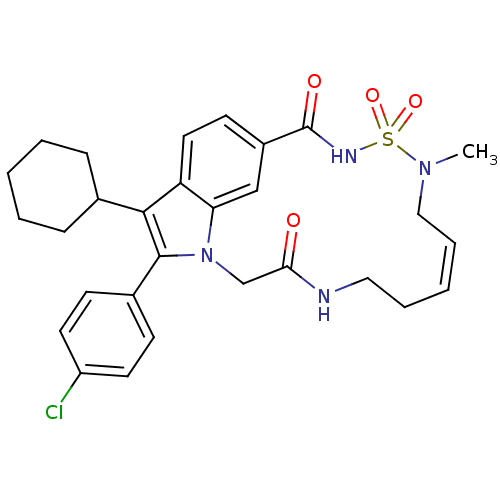
(US8524716, 20)Show SMILES CN1C\C=C/CCNC(=O)Cn2c(c(C3CCCCC3)c3ccc(cc23)C(=O)NS1(=O)=O)-c1ccc(Cl)cc1 |c:3| Show InChI InChI=1S/C29H33ClN4O4S/c1-33-17-7-3-6-16-31-26(35)19-34-25-18-22(29(36)32-39(33,37)38)12-15-24(25)27(20-8-4-2-5-9-20)28(34)21-10-13-23(30)14-11-21/h3,7,10-15,18,20H,2,4-6,8-9,16-17,19H2,1H3,(H,31,35)(H,32,36)/b7-3- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101318
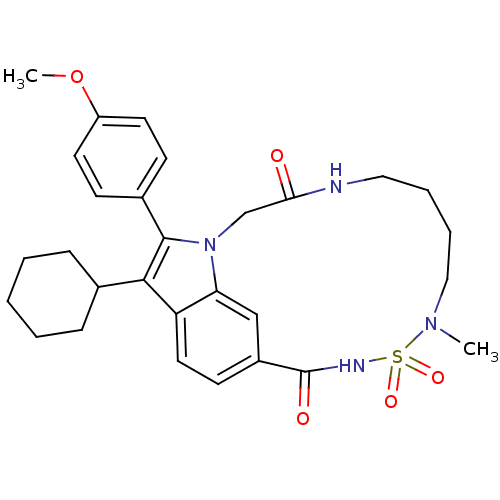
(US8524716, 25)Show SMILES COc1ccc(cc1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCCCCN(C)S(=O)(=O)NC3=O Show InChI InChI=1S/C29H36N4O5S/c1-32-17-7-6-16-30-26(34)19-33-25-18-22(29(35)31-39(32,36)37)12-15-24(25)27(20-8-4-3-5-9-20)28(33)21-10-13-23(38-2)14-11-21/h10-15,18,20H,3-9,16-17,19H2,1-2H3,(H,30,34)(H,31,35) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101314
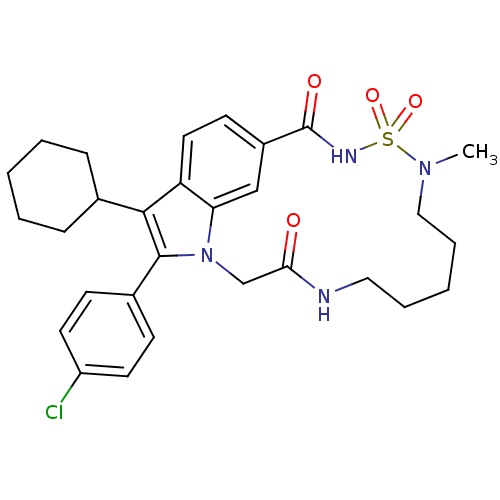
(US8524716, 21)Show SMILES CN1CCCCCNC(=O)Cn2c(c(C3CCCCC3)c3ccc(cc23)C(=O)NS1(=O)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C29H35ClN4O4S/c1-33-17-7-3-6-16-31-26(35)19-34-25-18-22(29(36)32-39(33,37)38)12-15-24(25)27(20-8-4-2-5-9-20)28(34)21-10-13-23(30)14-11-21/h10-15,18,20H,2-9,16-17,19H2,1H3,(H,31,35)(H,32,36) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101319

(US8524716, 210 | US8524716, 26)Show SMILES COc1ccc(cc1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCCCCCN(C)S(=O)(=O)NC3=O Show InChI InChI=1S/C30H38N4O5S/c1-33-18-8-4-7-17-31-27(35)20-34-26-19-23(30(36)32-40(33,37)38)13-16-25(26)28(21-9-5-3-6-10-21)29(34)22-11-14-24(39-2)15-12-22/h11-16,19,21H,3-10,17-18,20H2,1-2H3,(H,31,35)(H,32,36) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101327
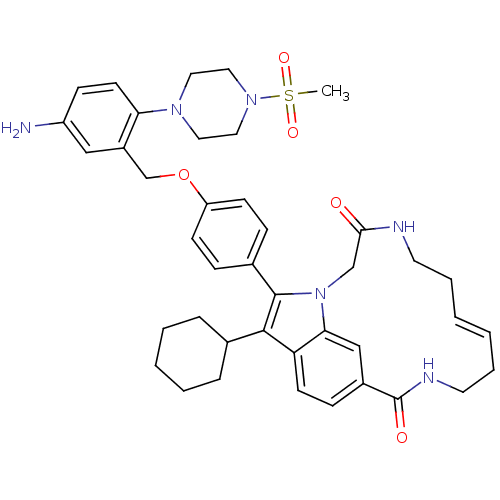
(US8524716, 4)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(N)cc1COc1ccc(cc1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCC\C=C/CCNC3=O |c:52| Show InChI InChI=1S/C41H50N6O5S/c1-53(50,51)46-23-21-45(22-24-46)36-18-14-33(42)25-32(36)28-52-34-15-11-30(12-16-34)40-39(29-9-5-4-6-10-29)35-17-13-31-26-37(35)47(40)27-38(48)43-19-7-2-3-8-20-44-41(31)49/h2-3,11-18,25-26,29H,4-10,19-24,27-28,42H2,1H3,(H,43,48)(H,44,49)/b3-2- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101328
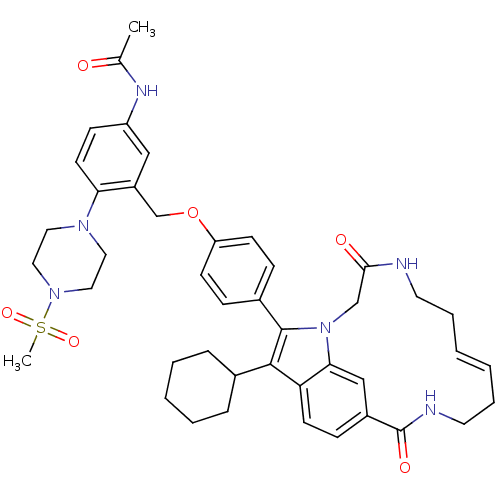
(US8524716, 5)Show SMILES CC(=O)Nc1ccc(N2CCN(CC2)S(C)(=O)=O)c(COc2ccc(cc2)-c2c(C3CCCCC3)c3ccc4cc3n2CC(=O)NCC\C=C/CCNC4=O)c1 |c:53| Show InChI InChI=1S/C43H52N6O6S/c1-30(50)46-35-15-19-38(47-22-24-48(25-23-47)56(2,53)54)34(26-35)29-55-36-16-12-32(13-17-36)42-41(31-10-6-5-7-11-31)37-18-14-33-27-39(37)49(42)28-40(51)44-20-8-3-4-9-21-45-43(33)52/h3-4,12-19,26-27,31H,5-11,20-25,28-29H2,1-2H3,(H,44,51)(H,45,52)(H,46,50)/b4-3- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101333

(US8524716, 12)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1COc1ccc(cc1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCC\C=C/CCNC3=O)N1CCCC1=O |c:51| Show InChI InChI=1S/C45H54N6O6S/c1-58(55,56)49-26-24-48(25-27-49)39-20-16-36(50-23-9-12-42(50)53)28-35(39)31-57-37-17-13-33(14-18-37)44-43(32-10-5-4-6-11-32)38-19-15-34-29-40(38)51(44)30-41(52)46-21-7-2-3-8-22-47-45(34)54/h2-3,13-20,28-29,32H,4-12,21-27,30-31H2,1H3,(H,46,52)(H,47,54)/b3-2- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101320
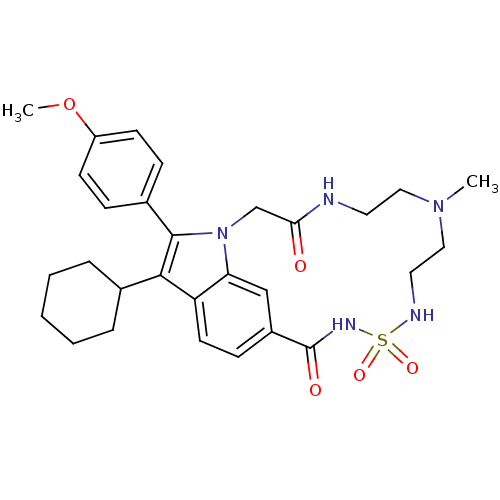
(US8524716, 27)Show SMILES COc1ccc(cc1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCCN(C)CCNS(=O)(=O)NC3=O Show InChI InChI=1S/C29H37N5O5S/c1-33-16-14-30-26(35)19-34-25-18-22(29(36)32-40(37,38)31-15-17-33)10-13-24(25)27(20-6-4-3-5-7-20)28(34)21-8-11-23(39-2)12-9-21/h8-13,18,20,31H,3-7,14-17,19H2,1-2H3,(H,30,35)(H,32,36) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101317
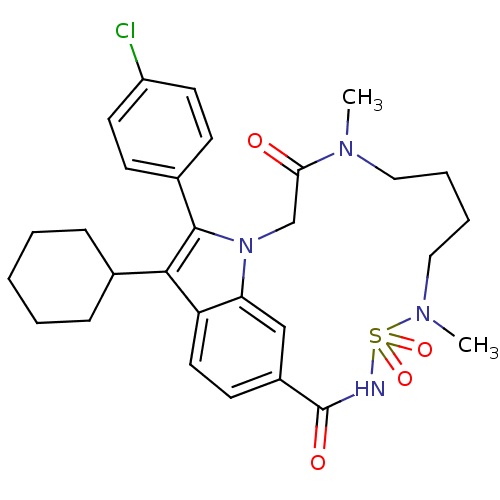
(US8524716, 24)Show SMILES CN1CCCCN(C)S(=O)(=O)NC(=O)c2ccc3c(C4CCCCC4)c(-c4ccc(Cl)cc4)n(CC1=O)c3c2 Show InChI InChI=1S/C29H35ClN4O4S/c1-32-16-6-7-17-33(2)39(37,38)31-29(36)22-12-15-24-25(18-22)34(19-26(32)35)28(21-10-13-23(30)14-11-21)27(24)20-8-4-3-5-9-20/h10-15,18,20H,3-9,16-17,19H2,1-2H3,(H,31,36) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101316
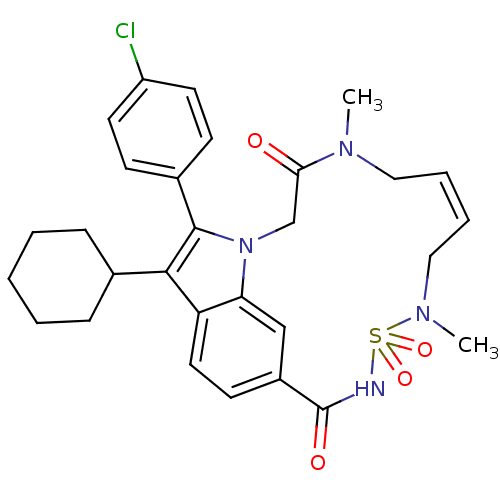
(US8524716, 23)Show SMILES CN1C\C=C/CN(C)S(=O)(=O)NC(=O)c2ccc3c(C4CCCCC4)c(-c4ccc(Cl)cc4)n(CC1=O)c3c2 |c:3| Show InChI InChI=1S/C29H33ClN4O4S/c1-32-16-6-7-17-33(2)39(37,38)31-29(36)22-12-15-24-25(18-22)34(19-26(32)35)28(21-10-13-23(30)14-11-21)27(24)20-8-4-3-5-9-20/h6-7,10-15,18,20H,3-5,8-9,16-17,19H2,1-2H3,(H,31,36)/b7-6- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101332
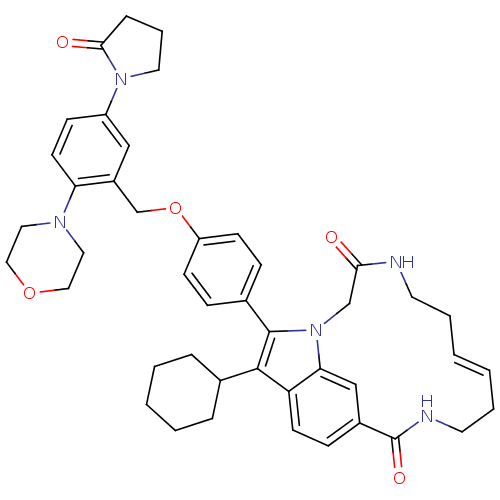
(US8524716, 11)Show SMILES O=C1CCCN1c1ccc(N2CCOCC2)c(COc2ccc(cc2)-c2c(C3CCCCC3)c3ccc4cc3n2CC(=O)NCC\C=C/CCNC4=O)c1 |c:52| Show InChI InChI=1S/C44H51N5O5/c50-40-29-49-39-28-33(44(52)46-21-7-2-1-6-20-45-40)14-18-37(39)42(31-9-4-3-5-10-31)43(49)32-12-16-36(17-13-32)54-30-34-27-35(48-22-8-11-41(48)51)15-19-38(34)47-23-25-53-26-24-47/h1-2,12-19,27-28,31H,3-11,20-26,29-30H2,(H,45,50)(H,46,52)/b2-1- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101335
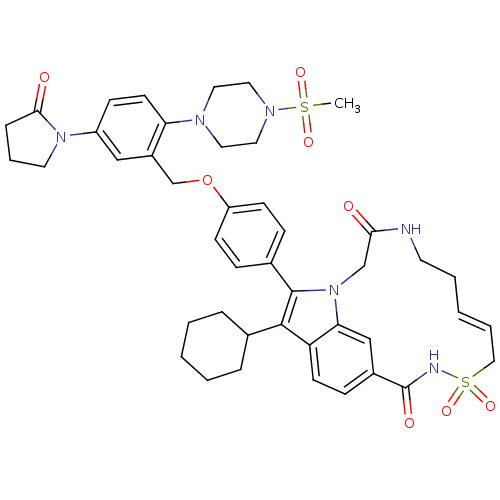
(US8524716, 14)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1COc1ccc(cc1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCC\C=C/CS(=O)(=O)NC3=O)N1CCCC1=O |c:51| Show InChI InChI=1S/C44H52N6O8S2/c1-59(54,55)48-24-22-47(23-25-48)38-19-15-35(49-21-8-11-41(49)52)27-34(38)30-58-36-16-12-32(13-17-36)43-42(31-9-4-2-5-10-31)37-18-14-33-28-39(37)50(43)29-40(51)45-20-6-3-7-26-60(56,57)46-44(33)53/h3,7,12-19,27-28,31H,2,4-6,8-11,20-26,29-30H2,1H3,(H,45,51)(H,46,53)/b7-3- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101326
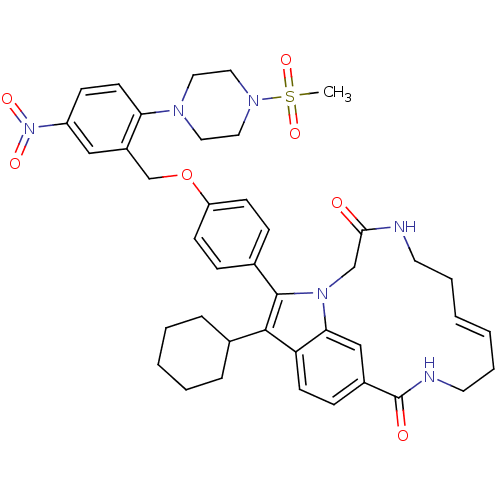
(US8524716, 3)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1COc1ccc(cc1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCC\C=C/CCNC3=O)N(=O)=O |c:51| Show InChI InChI=1S/C41H48N6O7S/c1-55(52,53)45-23-21-44(22-24-45)36-18-14-33(47(50)51)25-32(36)28-54-34-15-11-30(12-16-34)40-39(29-9-5-4-6-10-29)35-17-13-31-26-37(35)46(40)27-38(48)42-19-7-2-3-8-20-43-41(31)49/h2-3,11-18,25-26,29H,4-10,19-24,27-28H2,1H3,(H,42,48)(H,43,49)/b3-2- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101337
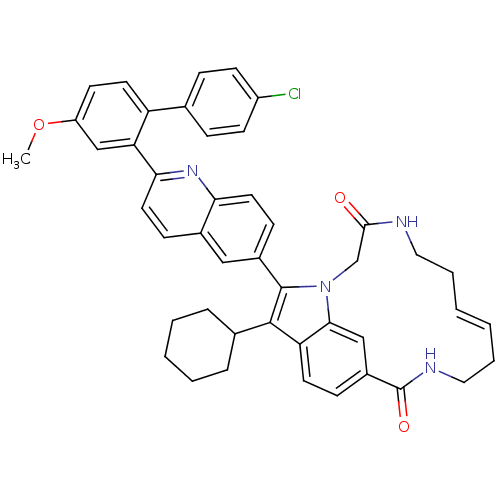
(US8524716, 16)Show SMILES COc1ccc(-c2ccc(Cl)cc2)c(c1)-c1ccc2cc(ccc2n1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCC\C=C/CCNC3=O |c:53| Show InChI InChI=1S/C45H43ClN4O3/c1-53-35-18-20-36(29-11-16-34(46)17-12-29)38(27-35)40-22-14-31-25-32(15-21-39(31)49-40)44-43(30-9-5-4-6-10-30)37-19-13-33-26-41(37)50(44)28-42(51)47-23-7-2-3-8-24-48-45(33)52/h2-3,11-22,25-27,30H,4-10,23-24,28H2,1H3,(H,47,51)(H,48,52)/b3-2- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101336
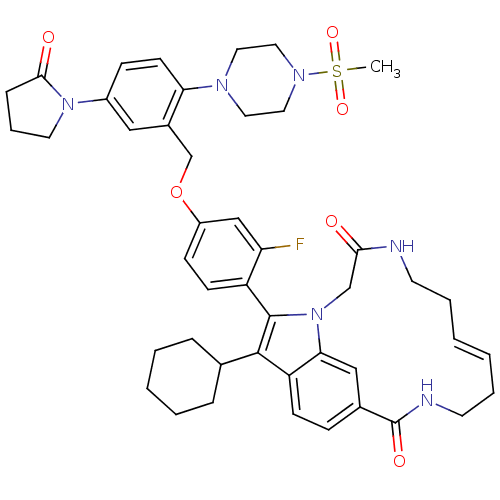
(US8524716, 15)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1COc1ccc(-c2c(C3CCCCC3)c3ccc4cc3n2CC(=O)NCC\C=C/CCNC4=O)c(F)c1)N1CCCC1=O |c:48,(2.66,9.57,;3.75,8.48,;2.21,8.48,;4.52,9.82,;4.52,7.15,;3.75,5.81,;4.52,4.48,;6.06,4.48,;6.83,5.81,;6.06,7.15,;6.83,3.15,;8.37,3.15,;9.14,1.81,;8.37,.48,;6.83,.48,;6.06,1.81,;4.52,1.81,;3.75,.48,;2.21,.48,;1.44,1.81,;-.1,1.81,;-.87,.48,;-2.41,.48,;-3.32,1.73,;-2.92,3.21,;-1.43,3.61,;-1.03,5.1,;-2.12,6.19,;-3.61,5.79,;-4.01,4.3,;-4.78,1.25,;-6.11,2.02,;-7.45,1.25,;-7.45,-.29,;-6.11,-1.06,;-4.78,-.29,;-3.32,-.77,;-2.92,-2.25,;-4.01,-3.34,;-3.61,-4.83,;-5.49,-2.94,;-6.58,-4.03,;-8.07,-3.63,;-9.16,-4.72,;-10.65,-4.32,;-9.88,-2.2,;-11.45,-1.06,;-10.12,-.29,;-8.78,-1.06,;-8.78,-2.6,;-.1,-.85,;-.87,-2.19,;1.44,-.85,;9.14,-.85,;10.58,-1.41,;10.5,-2.94,;9.01,-3.34,;8.17,-2.05,;6.63,-2.05,)| Show InChI InChI=1S/C45H53FN6O6S/c1-59(56,57)50-24-22-49(23-25-50)39-18-14-34(51-21-9-12-42(51)54)26-33(39)30-58-35-15-17-36(38(46)28-35)44-43(31-10-5-4-6-11-31)37-16-13-32-27-40(37)52(44)29-41(53)47-19-7-2-3-8-20-48-45(32)55/h2-3,13-18,26-28,31H,4-12,19-25,29-30H2,1H3,(H,47,53)(H,48,55)/b3-2- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101324
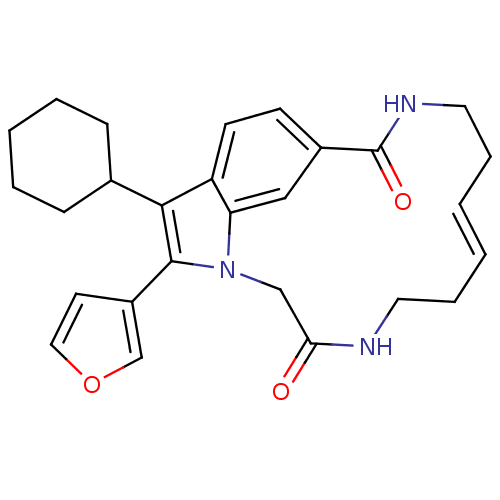
(US8524716, 1)Show SMILES O=C1Cn2c(-c3ccoc3)c(C3CCCCC3)c3ccc(cc23)C(=O)NCC\C=C/CCN1 |c:32| Show InChI InChI=1S/C27H31N3O3/c31-24-17-30-23-16-20(27(32)29-14-7-2-1-6-13-28-24)10-11-22(23)25(19-8-4-3-5-9-19)26(30)21-12-15-33-18-21/h1-2,10-12,15-16,18-19H,3-9,13-14,17H2,(H,28,31)(H,29,32)/b2-1- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101338
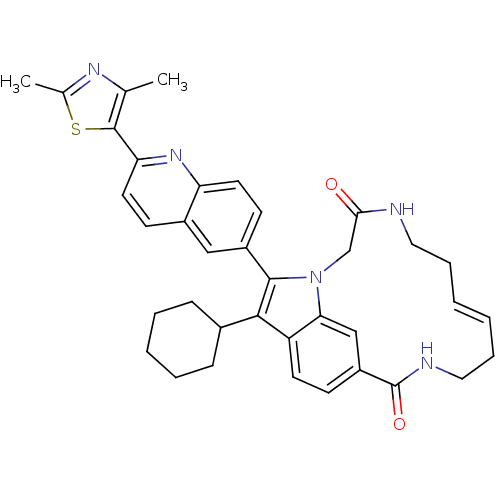
(US8524716, 17)Show SMILES Cc1nc(C)c(s1)-c1ccc2cc(ccc2n1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCC\C=C/CCNC3=O |c:44| Show InChI InChI=1S/C37H39N5O2S/c1-23-36(45-24(2)40-23)31-17-13-26-20-27(14-16-30(26)41-31)35-34(25-10-6-5-7-11-25)29-15-12-28-21-32(29)42(35)22-33(43)38-18-8-3-4-9-19-39-37(28)44/h3-4,12-17,20-21,25H,5-11,18-19,22H2,1-2H3,(H,38,43)(H,39,44)/b4-3- | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101329
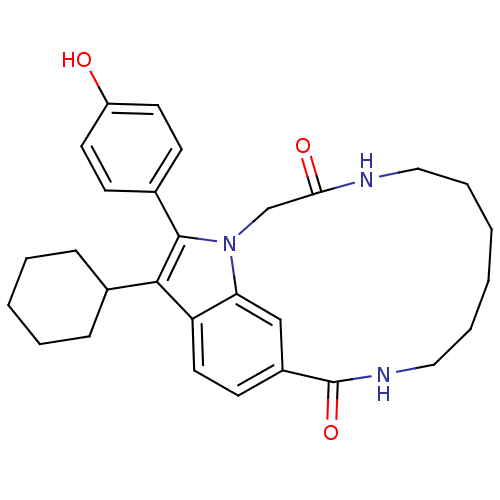
(US8524716, 8)Show SMILES Oc1ccc(cc1)-c1c(C2CCCCC2)c2ccc3cc2n1CC(=O)NCCCCCCNC3=O Show InChI InChI=1S/C29H35N3O3/c33-23-13-10-21(11-14-23)28-27(20-8-4-3-5-9-20)24-15-12-22-18-25(24)32(28)19-26(34)30-16-6-1-2-7-17-31-29(22)35/h10-15,18,20,33H,1-9,16-17,19H2,(H,30,34)(H,31,35) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101331
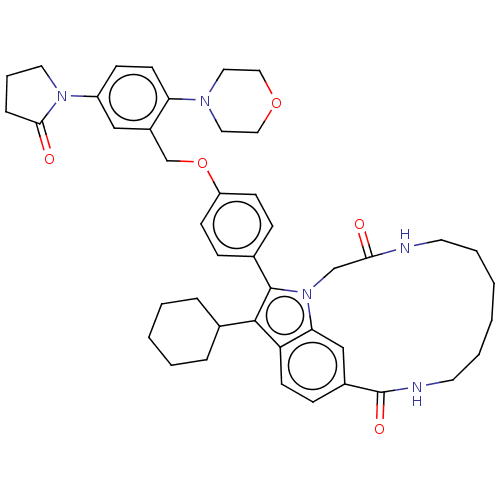
(US8524716, 10)Show SMILES O=C1CCCN1c1ccc(N2CCOCC2)c(COc2ccc(cc2)-c2c(C3CCCCC3)c3ccc4cc3n2CC(=O)NCCCCCCNC4=O)c1 Show InChI InChI=1S/C44H53N5O5/c50-40-29-49-39-28-33(44(52)46-21-7-2-1-6-20-45-40)14-18-37(39)42(31-9-4-3-5-10-31)43(49)32-12-16-36(17-13-32)54-30-34-27-35(48-22-8-11-41(48)51)15-19-38(34)47-23-25-53-26-24-47/h12-19,27-28,31H,1-11,20-26,29-30H2,(H,45,50)(H,46,52) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101325
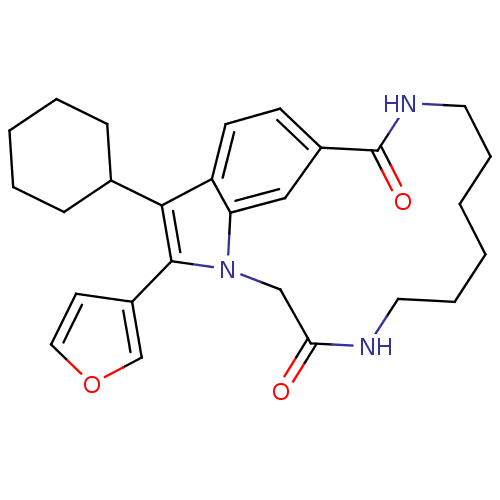
(US8524716, 2)Show SMILES O=C1Cn2c(-c3ccoc3)c(C3CCCCC3)c3ccc(cc23)C(=O)NCCCCCCN1 Show InChI InChI=1S/C27H33N3O3/c31-24-17-30-23-16-20(27(32)29-14-7-2-1-6-13-28-24)10-11-22(23)25(19-8-4-3-5-9-19)26(30)21-12-15-33-18-21/h10-12,15-16,18-19H,1-9,13-14,17H2,(H,28,31)(H,29,32) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101330

(US8524716, 9)Show SMILES COc1ccc(Br)c(COc2ccc(cc2)-c2c(C3CCCCC3)c3ccc4cc3n2CC(=O)NCCCCCCNC4=O)c1 Show InChI InChI=1S/C37H42BrN3O4/c1-44-30-16-18-32(38)28(21-30)24-45-29-14-11-26(12-15-29)36-35(25-9-5-4-6-10-25)31-17-13-27-22-33(31)41(36)23-34(42)39-19-7-2-3-8-20-40-37(27)43/h11-18,21-22,25H,2-10,19-20,23-24H2,1H3,(H,39,42)(H,40,43) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland
US Patent
| Assay Description
Inhibition assay using HCV NS5B. |
US Patent US8524716 (2013)
BindingDB Entry DOI: 10.7270/Q22R3Q9P |
More data for this
Ligand-Target Pair | |
DNA polymerase subunit gamma-1
(Homo sapiens (Human)) | BDBM50336503

(10-acetyl-11-(3-(benzyloxy)-4-methoxyphenyl)-3,3-d...)Show SMILES COc1ccc(cc1OCc1ccccc1)C1C2C(=O)CC(C)(C)CC2=Nc2ccccc2N1C(C)=O |c:28| Show InChI InChI=1S/C31H32N2O4/c1-20(34)33-25-13-9-8-12-23(25)32-24-17-31(2,3)18-26(35)29(24)30(33)22-14-15-27(36-4)28(16-22)37-19-21-10-6-5-7-11-21/h5-16,29-30H,17-19H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase gamma |
Antimicrob Agents Chemother 52: 4420-31 (2008)
Article DOI: 10.1128/AAC.00669-08
BindingDB Entry DOI: 10.7270/Q26M3732 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50336503

(10-acetyl-11-(3-(benzyloxy)-4-methoxyphenyl)-3,3-d...)Show SMILES COc1ccc(cc1OCc1ccccc1)C1C2C(=O)CC(C)(C)CC2=Nc2ccccc2N1C(C)=O |c:28| Show InChI InChI=1S/C31H32N2O4/c1-20(34)33-25-13-9-8-12-23(25)32-24-17-31(2,3)18-26(35)29(24)30(33)22-14-15-27(36-4)28(16-22)37-19-21-10-6-5-7-11-21/h5-16,29-30H,17-19H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha |
Antimicrob Agents Chemother 52: 4420-31 (2008)
Article DOI: 10.1128/AAC.00669-08
BindingDB Entry DOI: 10.7270/Q26M3732 |
More data for this
Ligand-Target Pair | |
DNA polymerase subunit gamma-1
(Homo sapiens (Human)) | BDBM50336502

(10-acetyl-11-(2,4-dichlorophenyl)-3,3-dimethyl-2,3...)Show SMILES CC(=O)N1C(C2C(=O)CC(C)(C)CC2=Nc2ccccc12)c1ccc(Cl)cc1Cl |c:14| Show InChI InChI=1S/C23H22Cl2N2O2/c1-13(28)27-19-7-5-4-6-17(19)26-18-11-23(2,3)12-20(29)21(18)22(27)15-9-8-14(24)10-16(15)25/h4-10,21-22H,11-12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase gamma |
Antimicrob Agents Chemother 52: 4420-31 (2008)
Article DOI: 10.1128/AAC.00669-08
BindingDB Entry DOI: 10.7270/Q26M3732 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50336502

(10-acetyl-11-(2,4-dichlorophenyl)-3,3-dimethyl-2,3...)Show SMILES CC(=O)N1C(C2C(=O)CC(C)(C)CC2=Nc2ccccc12)c1ccc(Cl)cc1Cl |c:14| Show InChI InChI=1S/C23H22Cl2N2O2/c1-13(28)27-19-7-5-4-6-17(19)26-18-11-23(2,3)12-20(29)21(18)22(27)15-9-8-14(24)10-16(15)25/h4-10,21-22H,11-12H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha |
Antimicrob Agents Chemother 52: 4420-31 (2008)
Article DOI: 10.1128/AAC.00669-08
BindingDB Entry DOI: 10.7270/Q26M3732 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50336502

(10-acetyl-11-(2,4-dichlorophenyl)-3,3-dimethyl-2,3...)Show SMILES CC(=O)N1C(C2C(=O)CC(C)(C)CC2=Nc2ccccc12)c1ccc(Cl)cc1Cl |c:14| Show InChI InChI=1S/C23H22Cl2N2O2/c1-13(28)27-19-7-5-4-6-17(19)26-18-11-23(2,3)12-20(29)21(18)22(27)15-9-8-14(24)10-16(15)25/h4-10,21-22H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta |
Antimicrob Agents Chemother 52: 4420-31 (2008)
Article DOI: 10.1128/AAC.00669-08
BindingDB Entry DOI: 10.7270/Q26M3732 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50336503

(10-acetyl-11-(3-(benzyloxy)-4-methoxyphenyl)-3,3-d...)Show SMILES COc1ccc(cc1OCc1ccccc1)C1C2C(=O)CC(C)(C)CC2=Nc2ccccc2N1C(C)=O |c:28| Show InChI InChI=1S/C31H32N2O4/c1-20(34)33-25-13-9-8-12-23(25)32-24-17-31(2,3)18-26(35)29(24)30(33)22-14-15-27(36-4)28(16-22)37-19-21-10-6-5-7-11-21/h5-16,29-30H,17-19H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta |
Antimicrob Agents Chemother 52: 4420-31 (2008)
Article DOI: 10.1128/AAC.00669-08
BindingDB Entry DOI: 10.7270/Q26M3732 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50336501

(10-acetyl-11-(4-(benzyloxy)-2-chlorophenyl)-6-hydr...)Show SMILES CC(=O)N1C(C2C(=O)CC(C)(C)CC2=Nc2c(O)cccc12)c1ccc(OCc2ccccc2)cc1Cl |c:14| Show InChI InChI=1S/C30H29ClN2O4/c1-18(34)33-24-10-7-11-25(35)28(24)32-23-15-30(2,3)16-26(36)27(23)29(33)21-13-12-20(14-22(21)31)37-17-19-8-5-4-6-9-19/h4-14,27,29,35H,15-17H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA
Curated by ChEMBL
| Assay Description
Inhibition of peripheral benzodiazepine BZD receptor by radioligand binding assay |
Antimicrob Agents Chemother 52: 4420-31 (2008)
Article DOI: 10.1128/AAC.00669-08
BindingDB Entry DOI: 10.7270/Q26M3732 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50336501

(10-acetyl-11-(4-(benzyloxy)-2-chlorophenyl)-6-hydr...)Show SMILES CC(=O)N1C(C2C(=O)CC(C)(C)CC2=Nc2c(O)cccc12)c1ccc(OCc2ccccc2)cc1Cl |c:14| Show InChI InChI=1S/C30H29ClN2O4/c1-18(34)33-24-10-7-11-25(35)28(24)32-23-15-30(2,3)16-26(36)27(23)29(33)21-13-12-20(14-22(21)31)37-17-19-8-5-4-6-9-19/h4-14,27,29,35H,15-17H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA
Curated by ChEMBL
| Assay Description
Inhibition of peripheral benzodiazepine BZD receptor by radioligand binding assay |
Antimicrob Agents Chemother 52: 4420-31 (2008)
Article DOI: 10.1128/AAC.00669-08
BindingDB Entry DOI: 10.7270/Q26M3732 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data