 Found 41 hits with Last Name = 'jung' and Initial = 'p'
Found 41 hits with Last Name = 'jung' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566082

(CHEMBL4779359)Show SMILES CN1CCN(Cc2ccc(-c3c[nH]c4ccncc34)c(OCCc3ccc(cc3)-c3cccc4ccncc34)c2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of probe-5 binding to human TNFalpha (77 to 233 residues) at 1 nM by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566088
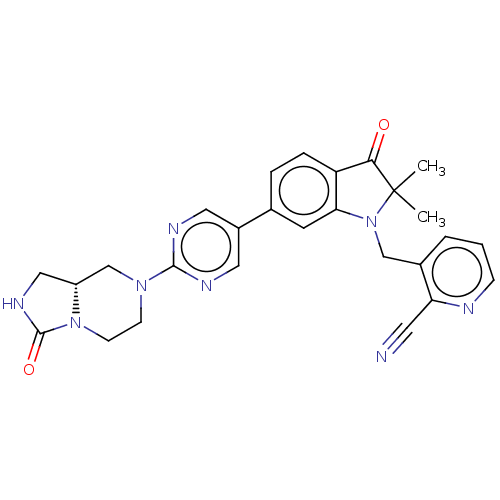
(CHEMBL4793924)Show SMILES [H][C@]12CNC(=O)N1CCN(C2)c1ncc(cn1)-c1ccc2C(=O)C(C)(C)N(Cc3cccnc3C#N)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of probe-5 binding to human TNFalpha (77 to 233 residues) at 1 nM by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Mus musculus) | BDBM50566088

(CHEMBL4793924)Show SMILES [H][C@]12CNC(=O)N1CCN(C2)c1ncc(cn1)-c1ccc2C(=O)C(C)(C)N(Cc3cccnc3C#N)c2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TNFalpha in mouse L929 cells exposed to compound incubated for 1 hr with mouse TNFalpha by luminescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Mus musculus) | BDBM50566087
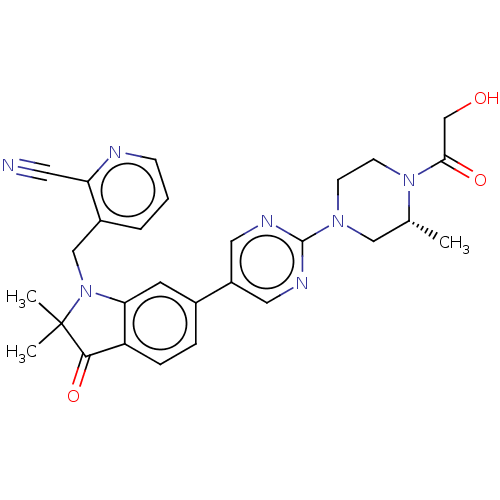
(CHEMBL4787790)Show SMILES C[C@@H]1CN(CCN1C(=O)CO)c1ncc(cn1)-c1ccc2C(=O)C(C)(C)N(Cc3cccnc3C#N)c2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TNFalpha in mouse L929 cells exposed to compound incubated for 1 hr with mouse TNFalpha by luminescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Mus musculus) | BDBM50566082

(CHEMBL4779359)Show SMILES CN1CCN(Cc2ccc(-c3c[nH]c4ccncc34)c(OCCc3ccc(cc3)-c3cccc4ccncc34)c2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TNFalpha in mouse L929 cells exposed to compound incubated for 1 hr with mouse TNFalpha by luminescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Mus musculus) | BDBM50566086

(CHEMBL4793441)Show SMILES C[C@@H]1CN(CCN1C(=O)CO)c1ncc(cn1)-c1ccc2C(=O)C(C)(C)N(Cc3ccccc3OC(F)F)c2c1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TNFalpha in mouse L929 cells exposed to compound incubated for 1 hr with mouse TNFalpha by luminescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566087

(CHEMBL4787790)Show SMILES C[C@@H]1CN(CCN1C(=O)CO)c1ncc(cn1)-c1ccc2C(=O)C(C)(C)N(Cc3cccnc3C#N)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of probe-5 binding to human TNFalpha (77 to 233 residues) at 1 nM by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566086

(CHEMBL4793441)Show SMILES C[C@@H]1CN(CCN1C(=O)CO)c1ncc(cn1)-c1ccc2C(=O)C(C)(C)N(Cc3ccccc3OC(F)F)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of probe-5 binding to human TNFalpha (77 to 233 residues) at 1 nM by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566081

(CHEMBL4778232)Show SMILES C(Cc1ccc(cc1)-c1cccc2ccncc12)Oc1ccccc1-c1cccc2ccncc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of probe-5 binding to human TNFalpha (77 to 233 residues) at 1 nM by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50570884
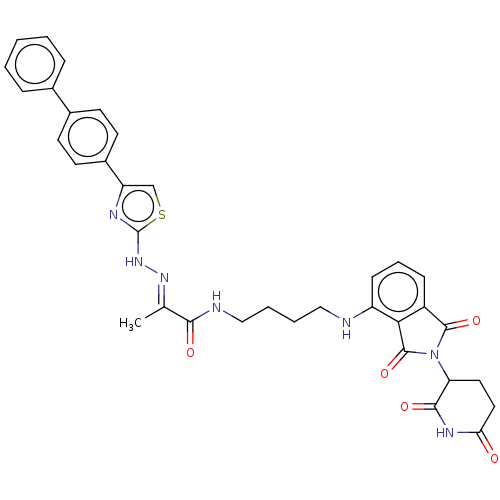
(CHEMBL4860669)Show SMILES C\C(=N/Nc1nc(cs1)-c1ccc(cc1)-c1ccccc1)C(=O)NCCCCNc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Cereblon in human Flp293T cells transfected with BRD4(BD2)-GFP fusion protein and mCherry reporter assessed as inhibition of dBET... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113435
BindingDB Entry DOI: 10.7270/Q2QV3R91 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566085
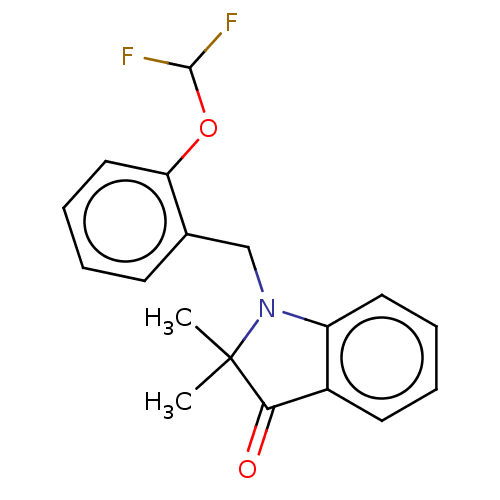
(CHEMBL4779860) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of probe-5 binding to human TNFalpha (77 to 233 residues) at 1 nM by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566080
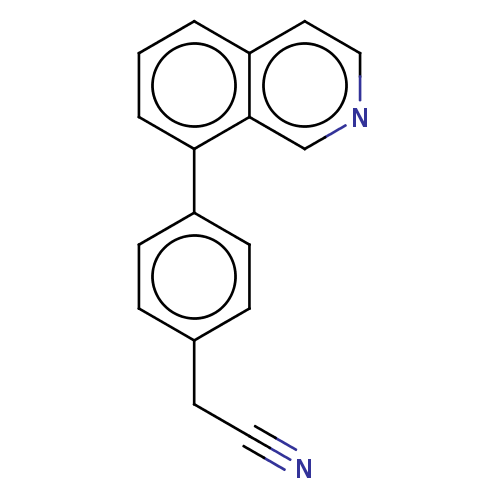
(CHEMBL4783333) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of probe-5 binding to human TNFalpha (77 to 233 residues) at 1 nM by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448109
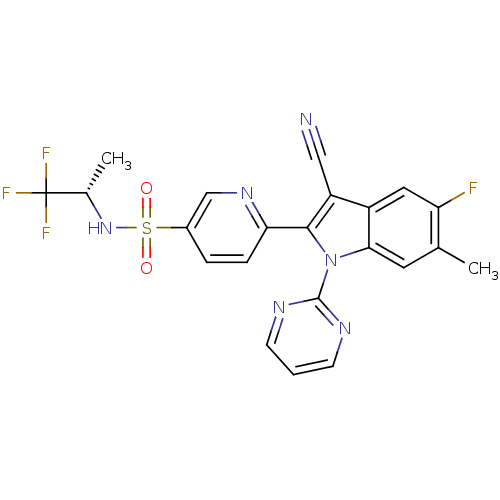
(CHEMBL3121811)Show SMILES C[C@H](NS(=O)(=O)c1ccc(nc1)-c1c(C#N)c2cc(F)c(C)cc2n1-c1ncccn1)C(F)(F)F |r| Show InChI InChI=1S/C22H16F4N6O2S/c1-12-8-19-15(9-17(12)23)16(10-27)20(32(19)21-28-6-3-7-29-21)18-5-4-14(11-30-18)35(33,34)31-13(2)22(24,25)26/h3-9,11,13,31H,1-2H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448108
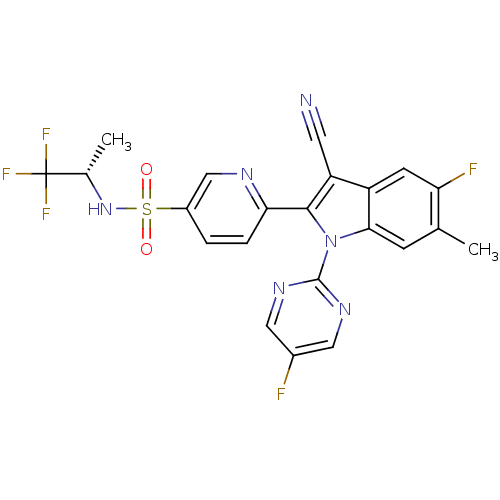
(CHEMBL3121694)Show SMILES C[C@H](NS(=O)(=O)c1ccc(nc1)-c1c(C#N)c2cc(F)c(C)cc2n1-c1ncc(F)cn1)C(F)(F)F |r| Show InChI InChI=1S/C22H15F5N6O2S/c1-11-5-19-15(6-17(11)24)16(7-28)20(33(19)21-30-8-13(23)9-31-21)18-4-3-14(10-29-18)36(34,35)32-12(2)22(25,26)27/h3-6,8-10,12,32H,1-2H3/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448107
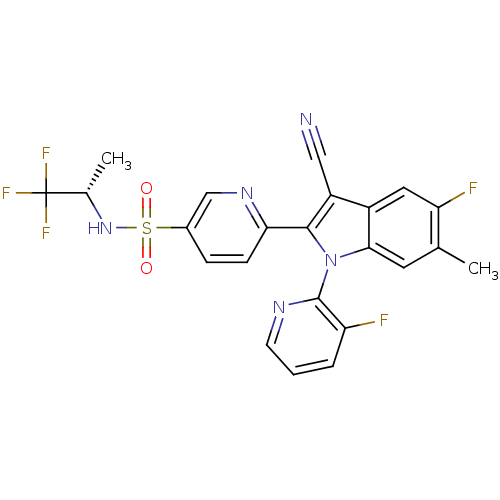
(CHEMBL3121798)Show SMILES C[C@H](NS(=O)(=O)c1ccc(nc1)-c1c(C#N)c2cc(F)c(C)cc2n1-c1ncccc1F)C(F)(F)F |r,wD:1.0,(62.21,-19.89,;61.43,-18.56,;59.89,-18.57,;59.13,-19.9,;59.14,-21.44,;60.47,-20.67,;57.6,-19.91,;56.81,-18.58,;55.27,-18.59,;54.52,-19.93,;55.29,-21.26,;56.83,-21.25,;52.98,-19.94,;52.07,-18.7,;52.53,-17.23,;53,-15.76,;50.6,-19.18,;49.27,-18.42,;47.94,-19.19,;46.6,-18.42,;47.94,-20.73,;46.6,-21.5,;49.27,-21.5,;50.61,-20.72,;52.08,-21.19,;52.85,-22.52,;54.38,-22.51,;55.15,-23.83,;54.38,-25.17,;52.85,-25.17,;52.08,-23.84,;50.54,-23.84,;62.2,-17.22,;63.74,-17.21,;61.42,-15.89,;62.95,-15.87,)| Show InChI InChI=1S/C23H16F5N5O2S/c1-12-8-20-15(9-18(12)25)16(10-29)21(33(20)22-17(24)4-3-7-30-22)19-6-5-14(11-31-19)36(34,35)32-13(2)23(26,27)28/h3-9,11,13,32H,1-2H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448106
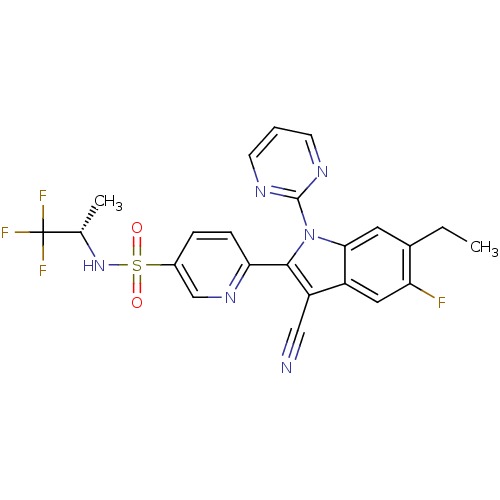
(CHEMBL3120505)Show SMILES CCc1cc2n(c(c(C#N)c2cc1F)-c1ccc(cn1)S(=O)(=O)N[C@@H](C)C(F)(F)F)-c1ncccn1 |r| Show InChI InChI=1S/C23H18F4N6O2S/c1-3-14-9-20-16(10-18(14)24)17(11-28)21(33(20)22-29-7-4-8-30-22)19-6-5-15(12-31-19)36(34,35)32-13(2)23(25,26)27/h4-10,12-13,32H,3H2,1-2H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448105
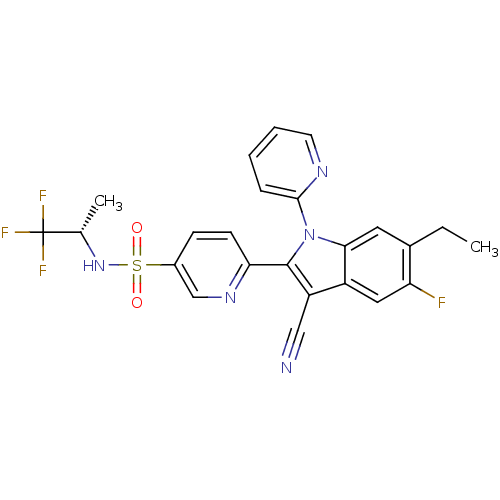
(CHEMBL3121800)Show SMILES CCc1cc2n(c(c(C#N)c2cc1F)-c1ccc(cn1)S(=O)(=O)N[C@@H](C)C(F)(F)F)-c1ccccn1 |r| Show InChI InChI=1S/C24H19F4N5O2S/c1-3-15-10-21-17(11-19(15)25)18(12-29)23(33(21)22-6-4-5-9-30-22)20-8-7-16(13-31-20)36(34,35)32-14(2)24(26,27)28/h4-11,13-14,32H,3H2,1-2H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448104
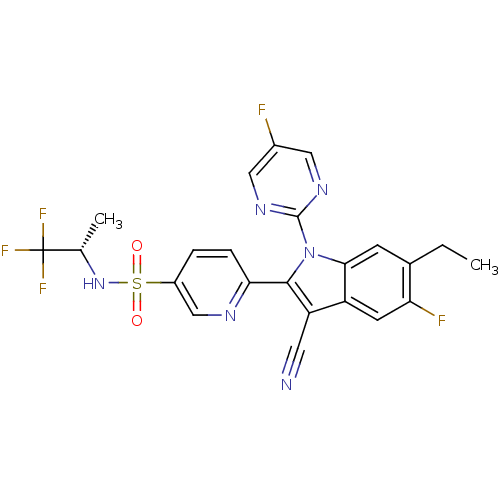
(CHEMBL3121804)Show SMILES CCc1cc2n(c(c(C#N)c2cc1F)-c1ccc(cn1)S(=O)(=O)N[C@@H](C)C(F)(F)F)-c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H17F5N6O2S/c1-3-13-6-20-16(7-18(13)25)17(8-29)21(34(20)22-31-9-14(24)10-32-22)19-5-4-15(11-30-19)37(35,36)33-12(2)23(26,27)28/h4-7,9-12,33H,3H2,1-2H3/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448109

(CHEMBL3121811)Show SMILES C[C@H](NS(=O)(=O)c1ccc(nc1)-c1c(C#N)c2cc(F)c(C)cc2n1-c1ncccn1)C(F)(F)F |r| Show InChI InChI=1S/C22H16F4N6O2S/c1-12-8-19-15(9-17(12)23)16(10-27)20(32(19)21-28-6-3-7-29-21)18-5-4-14(11-30-18)35(33,34)31-13(2)22(24,25)26/h3-9,11,13,31H,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448108

(CHEMBL3121694)Show SMILES C[C@H](NS(=O)(=O)c1ccc(nc1)-c1c(C#N)c2cc(F)c(C)cc2n1-c1ncc(F)cn1)C(F)(F)F |r| Show InChI InChI=1S/C22H15F5N6O2S/c1-11-5-19-15(6-17(11)24)16(7-28)20(33(19)21-30-8-13(23)9-31-21)18-4-3-14(10-29-18)36(34,35)32-12(2)22(25,26)27/h3-6,8-10,12,32H,1-2H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448107

(CHEMBL3121798)Show SMILES C[C@H](NS(=O)(=O)c1ccc(nc1)-c1c(C#N)c2cc(F)c(C)cc2n1-c1ncccc1F)C(F)(F)F |r,wD:1.0,(62.21,-19.89,;61.43,-18.56,;59.89,-18.57,;59.13,-19.9,;59.14,-21.44,;60.47,-20.67,;57.6,-19.91,;56.81,-18.58,;55.27,-18.59,;54.52,-19.93,;55.29,-21.26,;56.83,-21.25,;52.98,-19.94,;52.07,-18.7,;52.53,-17.23,;53,-15.76,;50.6,-19.18,;49.27,-18.42,;47.94,-19.19,;46.6,-18.42,;47.94,-20.73,;46.6,-21.5,;49.27,-21.5,;50.61,-20.72,;52.08,-21.19,;52.85,-22.52,;54.38,-22.51,;55.15,-23.83,;54.38,-25.17,;52.85,-25.17,;52.08,-23.84,;50.54,-23.84,;62.2,-17.22,;63.74,-17.21,;61.42,-15.89,;62.95,-15.87,)| Show InChI InChI=1S/C23H16F5N5O2S/c1-12-8-20-15(9-18(12)25)16(10-29)21(33(20)22-17(24)4-3-7-30-22)19-6-5-14(11-31-19)36(34,35)32-13(2)23(26,27)28/h3-9,11,13,32H,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448106

(CHEMBL3120505)Show SMILES CCc1cc2n(c(c(C#N)c2cc1F)-c1ccc(cn1)S(=O)(=O)N[C@@H](C)C(F)(F)F)-c1ncccn1 |r| Show InChI InChI=1S/C23H18F4N6O2S/c1-3-14-9-20-16(10-18(14)24)17(11-28)21(33(20)22-29-7-4-8-30-22)19-6-5-15(12-31-19)36(34,35)32-13(2)23(25,26)27/h4-10,12-13,32H,3H2,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448104

(CHEMBL3121804)Show SMILES CCc1cc2n(c(c(C#N)c2cc1F)-c1ccc(cn1)S(=O)(=O)N[C@@H](C)C(F)(F)F)-c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H17F5N6O2S/c1-3-13-6-20-16(7-18(13)25)17(8-29)21(34(20)22-31-9-14(24)10-32-22)19-5-4-15(11-30-19)37(35,36)33-12(2)23(26,27)28/h4-7,9-12,33H,3H2,1-2H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448105

(CHEMBL3121800)Show SMILES CCc1cc2n(c(c(C#N)c2cc1F)-c1ccc(cn1)S(=O)(=O)N[C@@H](C)C(F)(F)F)-c1ccccn1 |r| Show InChI InChI=1S/C24H19F4N5O2S/c1-3-15-10-21-17(11-19(15)25)18(12-29)23(33(21)22-6-4-5-9-30-22)20-8-7-16(13-31-20)36(34,35)32-14(2)24(26,27)28/h4-11,13-14,32H,3H2,1-2H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes |
J Med Chem 57: 2121-35 (2014)
Article DOI: 10.1021/jm401621g
BindingDB Entry DOI: 10.7270/Q2RR20RN |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50570885
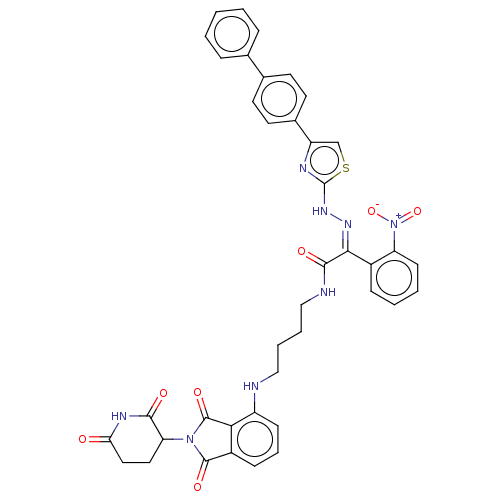
(CHEMBL4855690)Show SMILES [O-][N+](=O)c1ccccc1\C(=N\Nc1nc(cs1)-c1ccc(cc1)-c1ccccc1)C(=O)NCCCCNc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Cereblon in human Flp293T cells transfected with BRD4(BD2)-GFP fusion protein and mCherry reporter assessed as inhibition of dBET... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113435
BindingDB Entry DOI: 10.7270/Q2QV3R91 |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50570886

(CHEMBL4869528)Show SMILES C\C(=N/Nc1nc(c(CC(=O)NCCNc2cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c23)s1)-c1ccc(Cl)c(Cl)c1)C(=O)OCOC(=O)C(C)(C)C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Cereblon in human Flp293T cells transfected with BRD4(BD2)-GFP fusion protein and mCherry reporter assessed as inhibition of dBET... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113435
BindingDB Entry DOI: 10.7270/Q2QV3R91 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566079
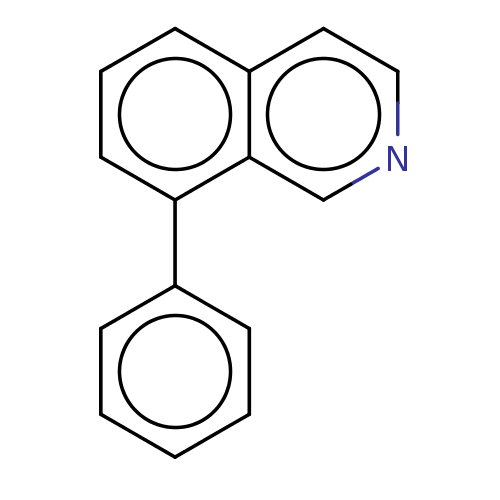
(CHEMBL4793685) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of probe-5 binding to human TNFalpha (77 to 233 residues) at 1 nM by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM50570883
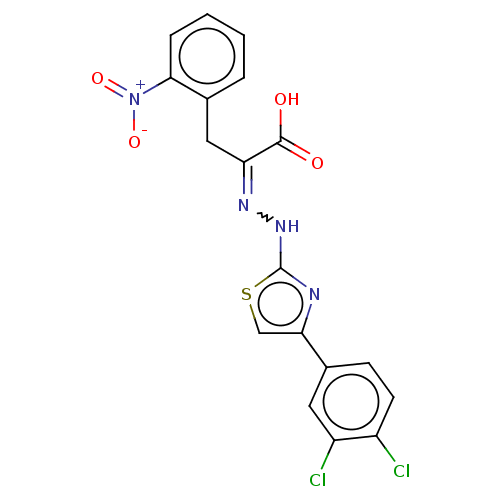
(CHEMBL4848695)Show SMILES OC(=O)C(Cc1ccccc1[N+]([O-])=O)=NNc1nc(cs1)-c1ccc(Cl)c(Cl)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of KKQYDREFLLDFQFK-FITCH from recombinant GST-tagged human eIF4E expressed in Escherichia coli BL21 (DE3) cells by fluorescence polariza... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113435
BindingDB Entry DOI: 10.7270/Q2QV3R91 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM50570882
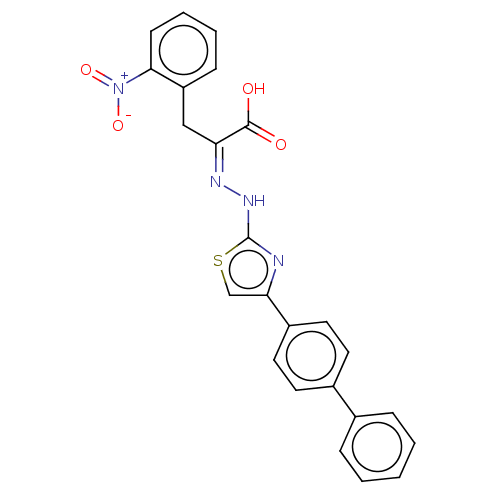
(CHEMBL3310358)Show SMILES OC(=O)C(\Cc1ccccc1[N+]([O-])=O)=N/Nc1nc(cs1)-c1ccc(cc1)-c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of KKQYDREFLLDFQFK-FITCH from recombinant GST-tagged human eIF4E expressed in Escherichia coli BL21 (DE3) cells by fluorescence polariza... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113435
BindingDB Entry DOI: 10.7270/Q2QV3R91 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM3787
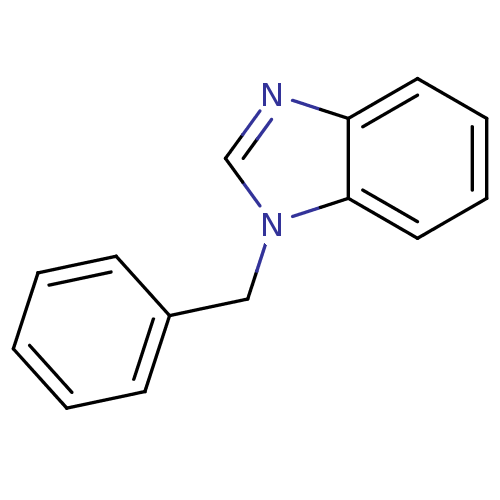
(1-Phenylbenzimidazole deriv. 4 | 1-benzyl-1H-1,3-b...)Show InChI InChI=1S/C14H12N2/c1-2-6-12(7-3-1)10-16-11-15-13-8-4-5-9-14(13)16/h1-9,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of probe-5 binding to human TNFalpha (77 to 233 residues) at 1 nM by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM3787

(1-Phenylbenzimidazole deriv. 4 | 1-benzyl-1H-1,3-b...)Show InChI InChI=1S/C14H12N2/c1-2-6-12(7-3-1)10-16-11-15-13-8-4-5-9-14(13)16/h1-9,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566082

(CHEMBL4779359)Show SMILES CN1CCN(Cc2ccc(-c3c[nH]c4ccncc34)c(OCCc3ccc(cc3)-c3cccc4ccncc34)c2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566081

(CHEMBL4778232)Show SMILES C(Cc1ccc(cc1)-c1cccc2ccncc12)Oc1ccccc1-c1cccc2ccncc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566080

(CHEMBL4783333) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566079

(CHEMBL4793685) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566083

(CHEMBL4777805) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566084
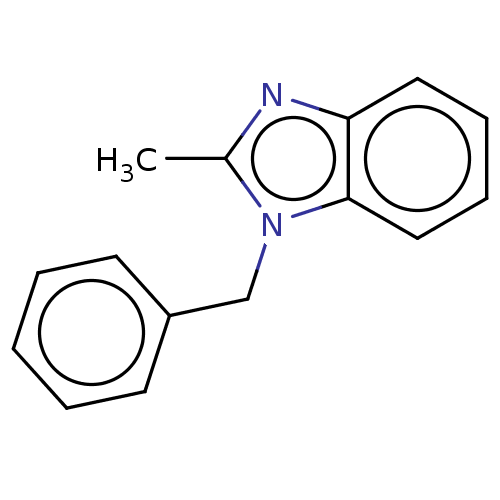
(CHEMBL1507048) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.25E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566085

(CHEMBL4779860) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566086

(CHEMBL4793441)Show SMILES C[C@@H]1CN(CCN1C(=O)CO)c1ncc(cn1)-c1ccc2C(=O)C(C)(C)N(Cc3ccccc3OC(F)F)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566088

(CHEMBL4793924)Show SMILES [H][C@]12CNC(=O)N1CCN(C2)c1ncc(cn1)-c1ccc2C(=O)C(C)(C)N(Cc3cccnc3C#N)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50566087

(CHEMBL4787790)Show SMILES C[C@@H]1CN(CCN1C(=O)CO)c1ncc(cn1)-c1ccc2C(=O)C(C)(C)N(Cc3cccnc3C#N)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated human TNFalpha trimer (77 to 233 residues) expressed in Escherichia coli BL21(DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01280
BindingDB Entry DOI: 10.7270/Q2DZ0D2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data