 Found 186 hits with Last Name = 'kumari' and Initial = 'p'
Found 186 hits with Last Name = 'kumari' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468040
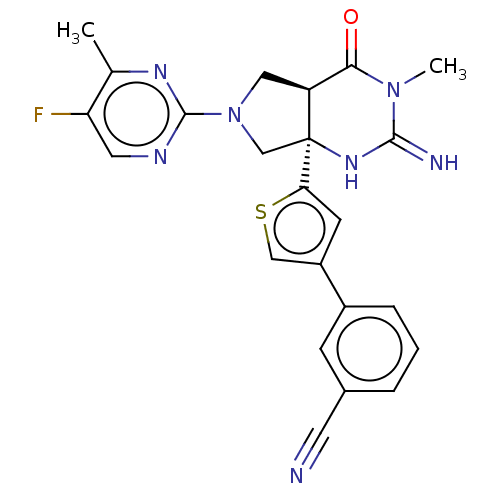
(CHEMBL4289763)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(C)n1 |r| Show InChI InChI=1S/C23H20FN7OS/c1-13-18(24)9-27-22(28-13)31-10-17-20(32)30(2)21(26)29-23(17,12-31)19-7-16(11-33-19)15-5-3-4-14(6-15)8-25/h3-7,9,11,17H,10,12H2,1-2H3,(H2,26,29)/t17-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468037
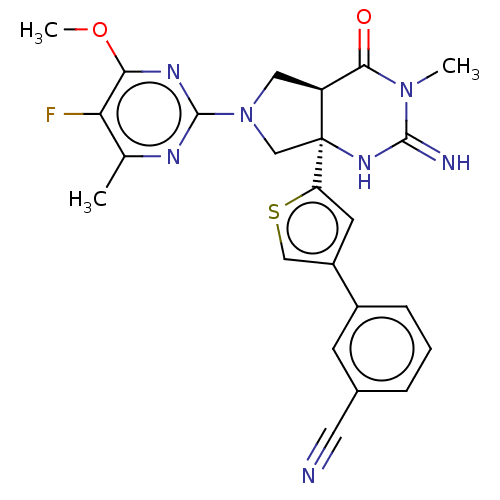
(CHEMBL4293298)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1nc(C)c(F)c(OC)n1 |r| Show InChI InChI=1S/C24H22FN7O2S/c1-13-19(25)20(34-3)29-23(28-13)32-10-17-21(33)31(2)22(27)30-24(17,12-32)18-8-16(11-35-18)15-6-4-5-14(7-15)9-26/h4-8,11,17H,10,12H2,1-3H3,(H2,27,30)/t17-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50164512
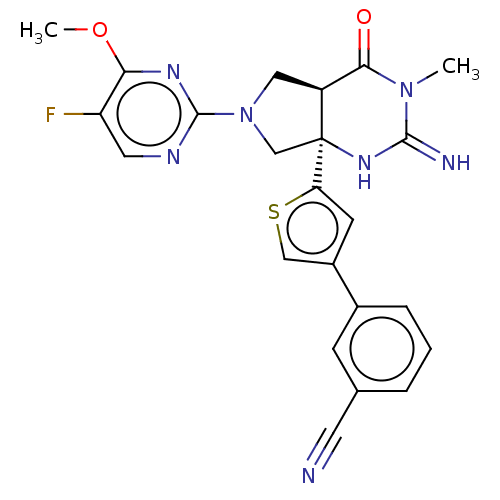
(CHEMBL3800286)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OC)n1 |r| Show InChI InChI=1S/C23H20FN7O2S/c1-30-20(32)16-10-31(22-27-9-17(24)19(28-22)33-2)12-23(16,29-21(30)26)18-7-15(11-34-18)14-5-3-4-13(6-14)8-25/h3-7,9,11,16H,10,12H2,1-2H3,(H2,26,29)/t16-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468043

(CHEMBL4288838)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1nc(C)c(F)c(C)n1 |r| Show InChI InChI=1S/C24H22FN7OS/c1-13-20(25)14(2)29-23(28-13)32-10-18-21(33)31(3)22(27)30-24(18,12-32)19-8-17(11-34-19)16-6-4-5-15(7-16)9-26/h4-8,11,18H,10,12H2,1-3H3,(H2,27,30)/t18-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468039

(CHEMBL4278329)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cc(OC)cc(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H20FN7O2S/c1-30-20(32)18-10-31(22-27-8-16(24)9-28-22)12-23(18,29-21(30)26)19-6-15(11-34-19)14-3-13(7-25)4-17(5-14)33-2/h3-6,8-9,11,18H,10,12H2,1-2H3,(H2,26,29)/t18-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468048

(CHEMBL4280271)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(Cl)c1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C21H18ClFN6OS/c1-28-18(30)16-9-29(20-25-7-15(23)8-26-20)11-21(16,27-19(28)24)17-6-13(10-31-17)12-3-2-4-14(22)5-12/h2-8,10,16H,9,11H2,1H3,(H2,24,27)/t16-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468038
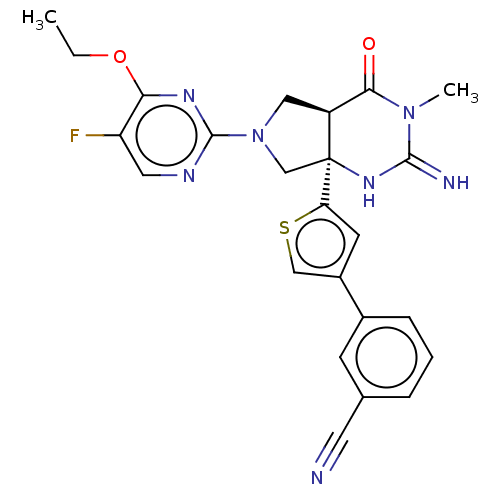
(CHEMBL4294236)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OCC)n1 |r| Show InChI InChI=1S/C24H22FN7O2S/c1-3-34-20-18(25)10-28-23(29-20)32-11-17-21(33)31(2)22(27)30-24(17,13-32)19-8-16(12-35-19)15-6-4-5-14(7-15)9-26/h4-8,10,12,17H,3,11,13H2,1-2H3,(H2,27,30)/t17-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468047

(CHEMBL4278011)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OCCC)n1 |r| Show InChI InChI=1S/C25H24FN7O2S/c1-3-7-35-21-19(26)11-29-24(30-21)33-12-18-22(34)32(2)23(28)31-25(18,14-33)20-9-17(13-36-20)16-6-4-5-15(8-16)10-27/h4-6,8-9,11,13,18H,3,7,12,14H2,1-2H3,(H2,28,31)/t18-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468046

(CHEMBL4278154)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(n1)N1CCOCC1 |r| Show InChI InChI=1S/C26H25FN8O2S/c1-33-23(36)19-13-35(25-30-12-20(27)22(31-25)34-5-7-37-8-6-34)15-26(19,32-24(33)29)21-10-18(14-38-21)17-4-2-3-16(9-17)11-28/h2-4,9-10,12,14,19H,5-8,13,15H2,1H3,(H2,29,32)/t19-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468044

(CHEMBL4279084)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(CC)n1 |r| Show InChI InChI=1S/C24H22FN7OS/c1-3-19-18(25)10-28-23(29-19)32-11-17-21(33)31(2)22(27)30-24(17,13-32)20-8-16(12-34-20)15-6-4-5-14(7-15)9-26/h4-8,10,12,17H,3,11,13H2,1-2H3,(H2,27,30)/t17-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468045

(CHEMBL4288644)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cc(F)cc(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H17F2N7OS/c1-30-19(32)17-9-31(21-27-7-16(24)8-28-21)11-22(17,29-20(30)26)18-5-14(10-33-18)13-2-12(6-25)3-15(23)4-13/h2-5,7-8,10,17H,9,11H2,1H3,(H2,26,29)/t17-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468035
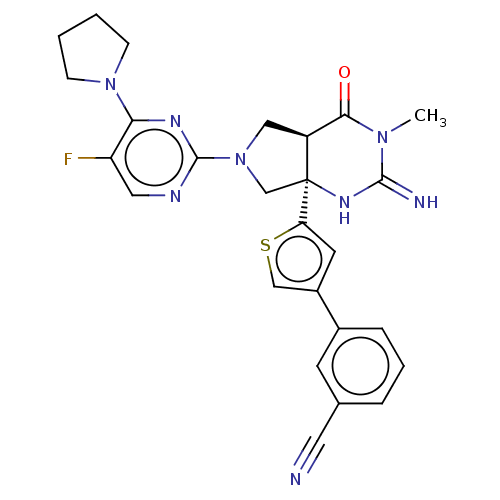
(CHEMBL4285940)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(n1)N1CCCC1 |r| Show InChI InChI=1S/C26H25FN8OS/c1-33-23(36)19-13-35(25-30-12-20(27)22(31-25)34-7-2-3-8-34)15-26(19,32-24(33)29)21-10-18(14-37-21)17-6-4-5-16(9-17)11-28/h4-6,9-10,12,14,19H,2-3,7-8,13,15H2,1H3,(H2,29,32)/t19-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468036

(CHEMBL4286732)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1ccc(F)c(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H17F2N7OS/c1-30-19(32)16-9-31(21-27-7-15(23)8-28-21)11-22(16,29-20(30)26)18-5-14(10-33-18)12-2-3-17(24)13(4-12)6-25/h2-5,7-8,10,16H,9,11H2,1H3,(H2,26,29)/t16-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398693
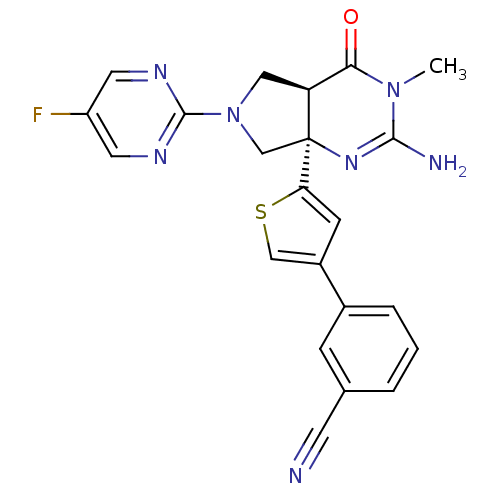
(CHEMBL2178718)Show SMILES CN1C(N)=N[C@]2(CN(C[C@H]2C1=O)c1ncc(F)cn1)c1cc(cs1)-c1cccc(c1)C#N |r,c:3| Show InChI InChI=1S/C22H18FN7OS/c1-29-19(31)17-10-30(21-26-8-16(23)9-27-21)12-22(17,28-20(29)25)18-6-15(11-32-18)14-4-2-3-13(5-14)7-24/h2-6,8-9,11,17H,10,12H2,1H3,(H2,25,28)/t17-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398687
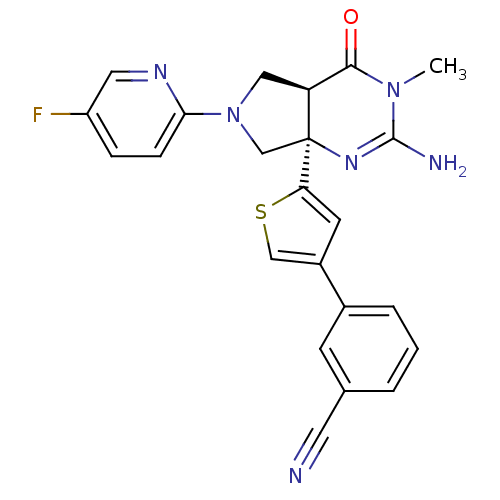
(CHEMBL2178714)Show SMILES CN1C(N)=N[C@]2(CN(C[C@H]2C1=O)c1ccc(F)cn1)c1cc(cs1)-c1cccc(c1)C#N |r,c:3| Show InChI InChI=1S/C23H19FN6OS/c1-29-21(31)18-11-30(20-6-5-17(24)10-27-20)13-23(18,28-22(29)26)19-8-16(12-32-19)15-4-2-3-14(7-15)9-25/h2-8,10,12,18H,11,13H2,1H3,(H2,26,28)/t18-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468042

(CHEMBL4294969)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1ccc(Cl)c(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H17ClFN7OS/c1-30-19(32)16-9-31(21-27-7-15(24)8-28-21)11-22(16,29-20(30)26)18-5-14(10-33-18)12-2-3-17(23)13(4-12)6-25/h2-5,7-8,10,16H,9,11H2,1H3,(H2,26,29)/t16-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468049
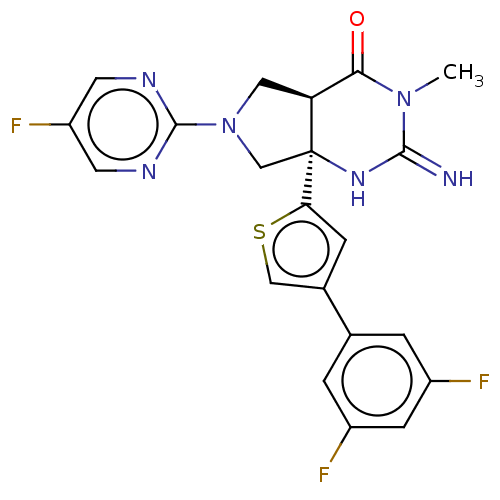
(CHEMBL4291347)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cc(F)cc(F)c1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C21H17F3N6OS/c1-29-18(31)16-8-30(20-26-6-15(24)7-27-20)10-21(16,28-19(29)25)17-4-12(9-32-17)11-2-13(22)5-14(23)3-11/h2-7,9,16H,8,10H2,1H3,(H2,25,28)/t16-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398696

(CHEMBL2178717)Show SMILES CN1C(N)=N[C@]2(CN(C[C@H]2C1=O)c1ncccn1)c1cc(cs1)-c1cccc(c1)C#N |r,c:3| Show InChI InChI=1S/C22H19N7OS/c1-28-19(30)17-11-29(21-25-6-3-7-26-21)13-22(17,27-20(28)24)18-9-16(12-31-18)15-5-2-4-14(8-15)10-23/h2-9,12,17H,11,13H2,1H3,(H2,24,27)/t17-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398680
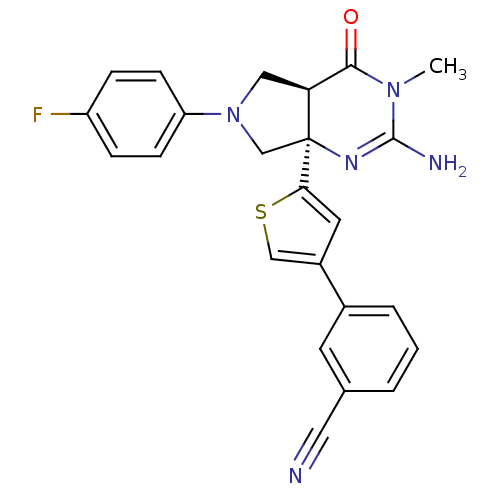
(CHEMBL2178150)Show SMILES CN1C(N)=N[C@]2(CN(C[C@H]2C1=O)c1ccc(F)cc1)c1cc(cs1)-c1cccc(c1)C#N |r,c:3| Show InChI InChI=1S/C24H20FN5OS/c1-29-22(31)20-12-30(19-7-5-18(25)6-8-19)14-24(20,28-23(29)27)21-10-17(13-32-21)16-4-2-3-15(9-16)11-26/h2-10,13,20H,12,14H2,1H3,(H2,27,28)/t20-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468041

(CHEMBL4282976)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(Br)cs1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C15H14BrFN6OS/c1-22-12(24)10-5-23(14-19-3-9(17)4-20-14)7-15(10,21-13(22)18)11-2-8(16)6-25-11/h2-4,6,10H,5,7H2,1H3,(H2,18,21)/t10-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50580628
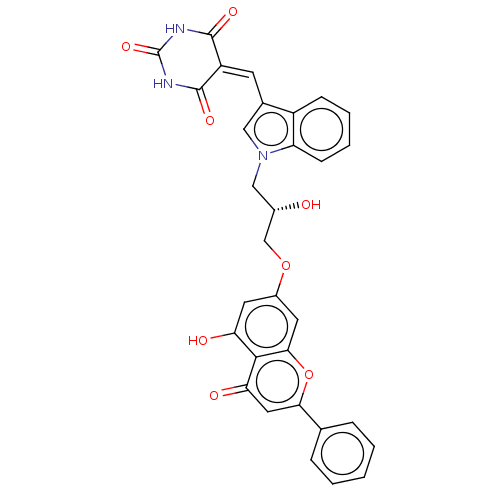
(CHEMBL5089355)Show SMILES [#8]-[#6@H](-[#6]-[#8]-c1cc(-[#8])c2c(c1)oc(cc2=O)-c1ccccc1)-[#6]-n1cc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX-2 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50580620
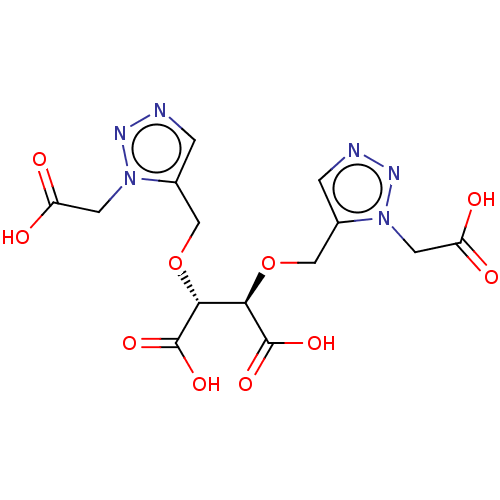
(CHEMBL5077595)Show SMILES OC(=O)Cn1nncc1CO[C@H]([C@@H](OCc1cnnn1CC(O)=O)C(O)=O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX-2 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13066
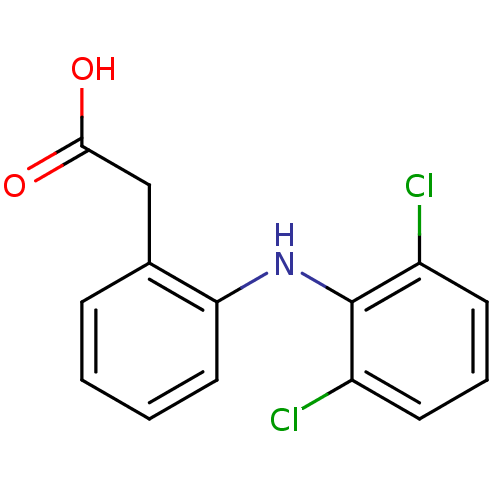
(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX-2 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13066

(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50539167
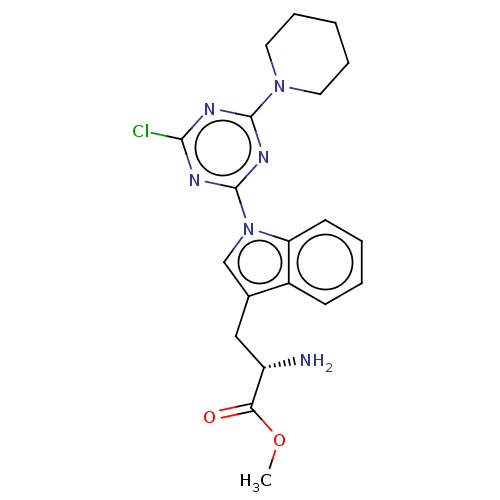
(CHEMBL4640256)Show SMILES COC(=O)[C@@H](N)Cc1cn(-c2nc(Cl)nc(n2)N2CCCCC2)c2ccccc12 |r| Show InChI InChI=1S/C20H23ClN6O2/c1-29-17(28)15(22)11-13-12-27(16-8-4-3-7-14(13)16)20-24-18(21)23-19(25-20)26-9-5-2-6-10-26/h3-4,7-8,12,15H,2,5-6,9-11,22H2,1H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50539165

(CHEMBL4640817)Show SMILES COC(=O)[C@@H](N)Cc1cn(-c2nc(Cl)nc(Nc3ccc(cc3)N3CCOCC3=O)n2)c2ccccc12 |r| Show InChI InChI=1S/C25H24ClN7O4/c1-36-22(35)19(27)12-15-13-33(20-5-3-2-4-18(15)20)25-30-23(26)29-24(31-25)28-16-6-8-17(9-7-16)32-10-11-37-14-21(32)34/h2-9,13,19H,10-12,14,27H2,1H3,(H,28,29,30,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50369123

(CHEMBL4170378)Show SMILES Oc1ccc(Nc2nc(Cl)nc(Nc3ccc(cc3)N3CCOCC3=O)n2)cc1 Show InChI InChI=1S/C19H17ClN6O3/c20-17-23-18(25-19(24-17)22-13-3-7-15(27)8-4-13)21-12-1-5-14(6-2-12)26-9-10-29-11-16(26)28/h1-8,27H,9-11H2,(H2,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 pre-incubated for 10 mins before arachidonic acid addition and measured after 2 mins by EIA method |
J Med Chem 61: 7929-7941 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00922
BindingDB Entry DOI: 10.7270/Q20004NS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX-2 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 pre-incubated for 10 mins before arachidonic acid addition and measured after 2 mins by EIA method |
J Med Chem 61: 7929-7941 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00922
BindingDB Entry DOI: 10.7270/Q20004NS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50369080

(CHEMBL4167700)Show InChI InChI=1S/C17H19ClN6O2/c18-15-20-16(22-17(21-15)23-7-1-2-8-23)19-12-3-5-13(6-4-12)24-9-10-26-11-14(24)25/h3-6H,1-2,7-11H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 pre-incubated for 10 mins before arachidonic acid addition and measured after 2 mins by EIA method |
J Med Chem 61: 7929-7941 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00922
BindingDB Entry DOI: 10.7270/Q20004NS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50369080

(CHEMBL4167700)Show InChI InChI=1S/C17H19ClN6O2/c18-15-20-16(22-17(21-15)23-7-1-2-8-23)19-12-3-5-13(6-4-12)24-9-10-26-11-14(24)25/h3-6H,1-2,7-11H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM13066

(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM13066

(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 pre-incubated for 10 mins before arachidonic acid addition and measured after 2 mins by EIA method |
J Med Chem 61: 7929-7941 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00922
BindingDB Entry DOI: 10.7270/Q20004NS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50369131

(CHEMBL4165902)Show SMILES Clc1ccc(Nc2nc(Cl)nc(Nc3ccc(cc3)N3CCOCC3=O)n2)cc1 Show InChI InChI=1S/C19H16Cl2N6O2/c20-12-1-3-13(4-2-12)22-18-24-17(21)25-19(26-18)23-14-5-7-15(8-6-14)27-9-10-29-11-16(27)28/h1-8H,9-11H2,(H2,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 pre-incubated for 10 mins before arachidonic acid addition and measured after 2 mins by EIA method |
J Med Chem 61: 7929-7941 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00922
BindingDB Entry DOI: 10.7270/Q20004NS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50369122

(CHEMBL4165753)Show SMILES Clc1nc(Nc2ccccc2)nc(Nc2ccc(cc2)N2CCOCC2=O)n1 Show InChI InChI=1S/C19H17ClN6O2/c20-17-23-18(21-13-4-2-1-3-5-13)25-19(24-17)22-14-6-8-15(9-7-14)26-10-11-28-12-16(26)27/h1-9H,10-12H2,(H2,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 pre-incubated for 10 mins before arachidonic acid addition and measured after 2 mins by EIA method |
J Med Chem 61: 7929-7941 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00922
BindingDB Entry DOI: 10.7270/Q20004NS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50539170

(CHEMBL4640306)Show InChI InChI=1S/C17H18ClN7O2/c18-15-19-16(24-6-8-26-9-7-24)21-17(20-15)27-12-14-11-25(23-22-14)10-13-4-2-1-3-5-13/h1-5,11H,6-10,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50539168

(CHEMBL4636792)Show SMILES N[C@@H](Cc1cn(-c2nc(Cl)nc(n2)N2CCOCC2)c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C18H19ClN6O3/c19-16-21-17(24-5-7-28-8-6-24)23-18(22-16)25-10-11(9-13(20)15(26)27)12-3-1-2-4-14(12)25/h1-4,10,13H,5-9,20H2,(H,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50580610

(CHEMBL5079967)Show SMILES CCOC(=O)Cn1nncc1CO[C@H]([C@@H](OCc1cnnn1CC(=O)OCC)C(=O)OCC)C(=O)OCC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX-2 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50539174

(CHEMBL4634582)Show SMILES COC(=O)[C@H](Cc1ccccc1)n1cc(COc2nc(Cl)nc(n2)N2CCOCC2)nn1 |r| Show InChI InChI=1S/C20H22ClN7O4/c1-30-17(29)16(11-14-5-3-2-4-6-14)28-12-15(25-26-28)13-32-20-23-18(21)22-19(24-20)27-7-9-31-10-8-27/h2-6,12,16H,7-11,13H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 10 mins followed by substrate addition and measured after 2 mins by EIA as... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115246
BindingDB Entry DOI: 10.7270/Q2H70KCN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50038649
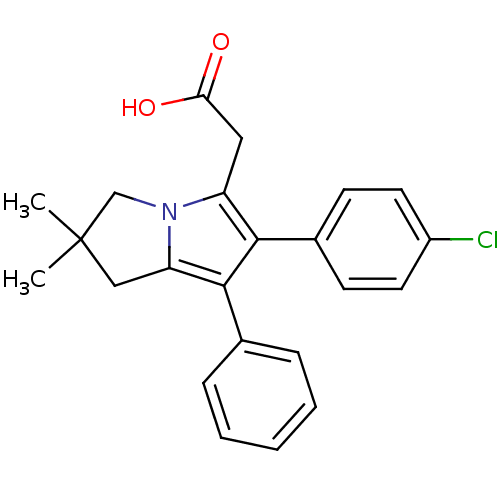
(2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...)Show SMILES CC1(C)Cc2c(c(c(CC(O)=O)n2C1)-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C23H22ClNO2/c1-23(2)13-19-22(15-6-4-3-5-7-15)21(16-8-10-17(24)11-9-16)18(12-20(26)27)25(19)14-23/h3-11H,12-14H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50580629

(CHEMBL5085564)Show SMILES COc1cc(cc(OC)c1OC)-c1cc(nn1-c1ccccc1)C(=O)CCCOc1ccc2ccc(=O)oc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX-2 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50580620

(CHEMBL5077595)Show SMILES OC(=O)Cn1nncc1CO[C@H]([C@@H](OCc1cnnn1CC(O)=O)C(O)=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50580628

(CHEMBL5089355)Show SMILES [#8]-[#6@H](-[#6]-[#8]-c1cc(-[#8])c2c(c1)oc(cc2=O)-c1ccccc1)-[#6]-n1cc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50580611

(CHEMBL5089124)Show SMILES CCOC(=O)[C@H](OCc1cnnn1[C@@H](CO)C(=O)OC)[C@@H](OCc1cnnn1[C@@H](CO)C(=O)OC)C(=O)OCC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX-2 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50580615

(CHEMBL5084816)Show SMILES CCOC(=O)[C@H](OCc1cnnn1[C@@H](Cc1c[nH]c2ccccc12)C(=O)OC)[C@@H](OCc1cnnn1[C@@H](Cc1c[nH]c2ccccc12)C(=O)OC)C(=O)OCC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX-2 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50369081

(CHEMBL4172724)Show SMILES Clc1nc(Nc2ccc(Br)cc2)nc(Nc2ccc(cc2)N2CCOCC2=O)n1 Show InChI InChI=1S/C19H16BrClN6O2/c20-12-1-3-13(4-2-12)22-18-24-17(21)25-19(26-18)23-14-5-7-15(8-6-14)27-9-10-29-11-16(27)28/h1-8H,9-11H2,(H2,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 pre-incubated for 10 mins before arachidonic acid addition and measured after 2 mins by EIA method |
J Med Chem 61: 7929-7941 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00922
BindingDB Entry DOI: 10.7270/Q20004NS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data