

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 1 (Bos taurus) | BDBM50038649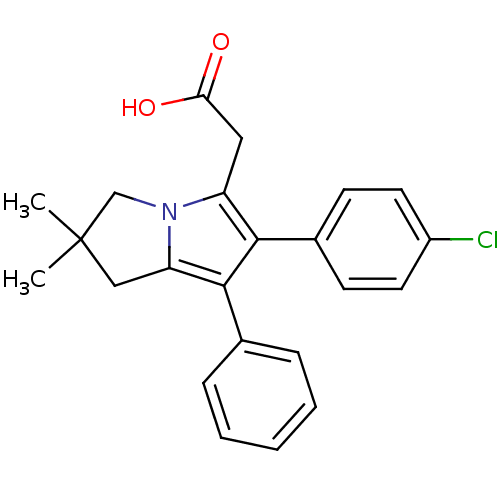 (2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Inhibition of bovine COX1 assessed as inhibition of calcium ionophore A23187-induced 12-hydroxyheptadecatrienoic acid formation by reverse-phase HPLC... | Bioorg Med Chem Lett 22: 5031-4 (2012) Article DOI: 10.1016/j.bmcl.2012.06.012 BindingDB Entry DOI: 10.7270/Q27P90GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50038649 (2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against prostaglandin G/H synthase 1 | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50038649 (2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 preincubated for 5 mins followed by arachidonic acid substrate addition by colorimetric enzyme immunoassay | Bioorg Med Chem 25: 4424-4432 (2017) Article DOI: 10.1016/j.bmc.2017.06.027 BindingDB Entry DOI: 10.7270/Q2X92DRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Bos taurus) | BDBM50038649 (2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University Curated by ChEMBL | Assay Description Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC | Bioorg Med Chem 17: 558-68 (2009) Article DOI: 10.1016/j.bmc.2008.11.074 BindingDB Entry DOI: 10.7270/Q2X92B4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50038649 (2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50038649 (2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00880 BindingDB Entry DOI: 10.7270/Q2JH3R2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50038649 (2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bhupat and Jyoti Mehta School of Biosciences Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) | Eur J Med Chem 124: 428-434 (2016) Article DOI: 10.1016/j.ejmech.2016.08.066 BindingDB Entry DOI: 10.7270/Q2639RRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50038649 (2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated for 10 mins followed by substr... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112066 BindingDB Entry DOI: 10.7270/Q29C722K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||