 Found 167 hits with Last Name = 'parikh' and Initial = 'p'
Found 167 hits with Last Name = 'parikh' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50608974

(CHEMBL5288856)Show SMILES Cc1nc2ccccc2nc1-c1cc2nc(cc(N([11CH3])C3CCOCC3)n2n1)N1CCC(F)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50608972

(CHEMBL5266334)Show SMILES Cc1nc2ccccc2nc1\C=C\c1nc(NC2CCOCC2)cc(n1)N1CCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591018
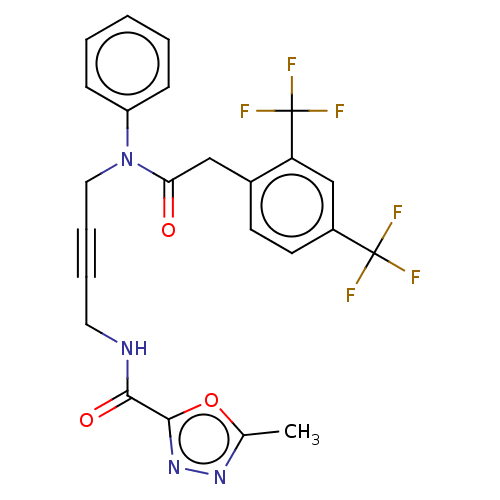
(CHEMBL5204203)Show SMILES Cc1nnc(o1)C(=O)NCC#CCN(C(=O)Cc1ccc(cc1C(F)(F)F)C(F)(F)F)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50165469

(CHEMBL3797911)Show SMILES COc1ccc2c(OCc3nnc4ncc(nn34)-c3ccccc3)ccnc2c1 Show InChI InChI=1S/C21H16N6O2/c1-28-15-7-8-16-17(11-15)22-10-9-19(16)29-13-20-24-25-21-23-12-18(26-27(20)21)14-5-3-2-4-6-14/h2-12H,13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50608973

(CHEMBL4563588)Show SMILES Cc1nc2ccc(F)cc2nc1\C=C\c1nc(NC2CCOCC2)cc(n1)N1CCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50035620

(CHEMBL3338445)Show SMILES COc1ccc(CNc2nc(OCc3ccccn3)ncc2C(=O)c2cc(OC)c(OC)c(OC)c2)cc1Cl Show InChI InChI=1S/C28H27ClN4O6/c1-35-22-9-8-17(11-21(22)29)14-31-27-20(15-32-28(33-27)39-16-19-7-5-6-10-30-19)25(34)18-12-23(36-2)26(38-4)24(13-18)37-3/h5-13,15H,14,16H2,1-4H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591016
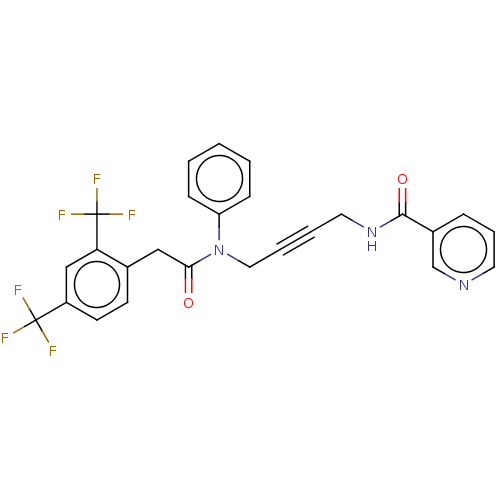
(CHEMBL5175156)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#CCNC(=O)c2cccnc2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50608971
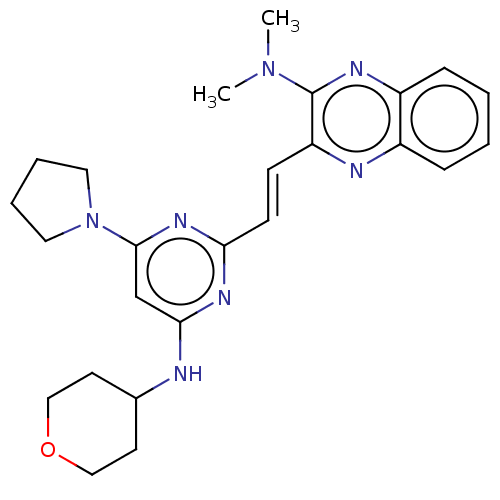
(CHEMBL5290355)Show SMILES CN(C)c1nc2ccccc2nc1\C=C\c1nc(NC2CCOCC2)cc(n1)N1CCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591017
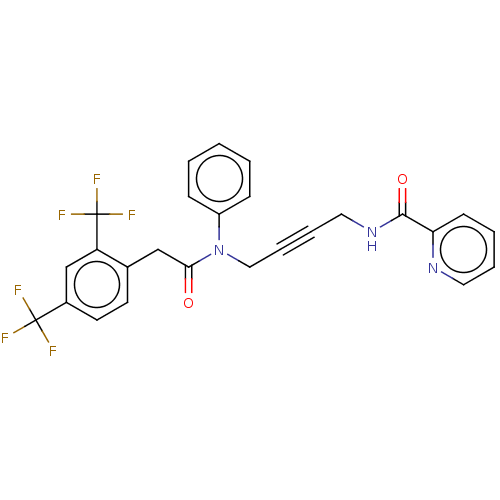
(CHEMBL5206022)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#CCNC(=O)c2ccccn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448084
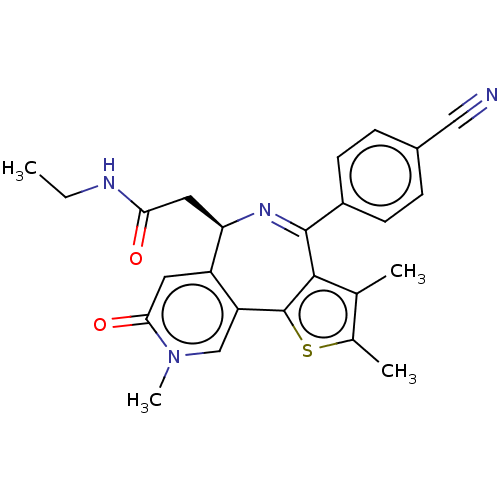
(US10689395, Compound k | US11267820, Compound k)Show SMILES CCNC(=O)C[C@H]1N=C(c2c(C)c(C)sc2-c2cn(C)c(=O)cc12)c1ccc(cc1)C#N |c:7| Show InChI InChI=1S/C25H24N4O2S/c1-5-27-21(30)11-20-18-10-22(31)29(4)13-19(18)25-23(14(2)15(3)32-25)24(28-20)17-8-6-16(12-26)7-9-17/h6-10,13,20H,5,11H2,1-4H3,(H,27,30)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED
US Patent
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
US Patent US10689395 (2020)
BindingDB Entry DOI: 10.7270/Q21J9DTK |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448084

(US10689395, Compound k | US11267820, Compound k)Show SMILES CCNC(=O)C[C@H]1N=C(c2c(C)c(C)sc2-c2cn(C)c(=O)cc12)c1ccc(cc1)C#N |c:7| Show InChI InChI=1S/C25H24N4O2S/c1-5-27-21(30)11-20-18-10-22(31)29(4)13-19(18)25-23(14(2)15(3)32-25)24(28-20)17-8-6-16(12-26)7-9-17/h6-10,13,20H,5,11H2,1-4H3,(H,27,30)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H1X |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50608964

(CHEMBL5271175)Show SMILES Cc1cc(-c2ccncc2)n(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591022
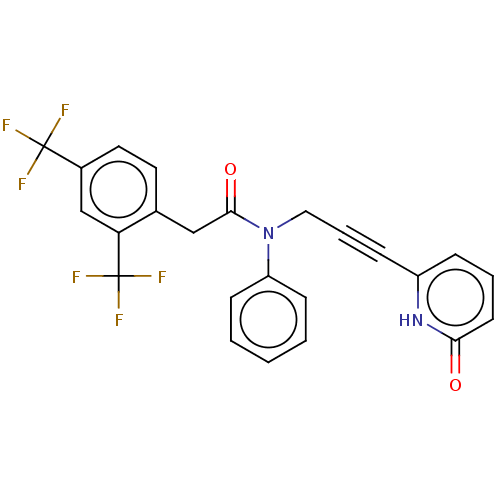
(CHEMBL5197367)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2cccc(=O)[nH]2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591035
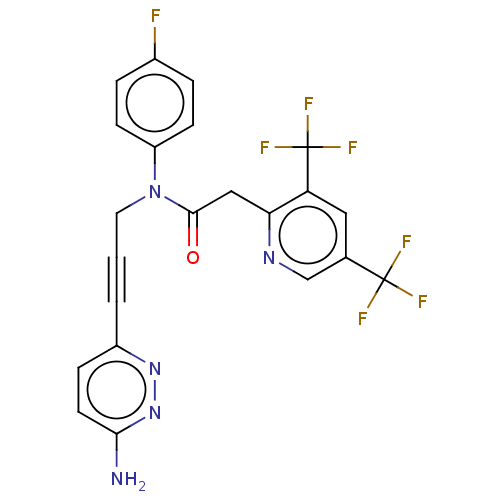
(CHEMBL5187422)Show SMILES Nc1ccc(nn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591030
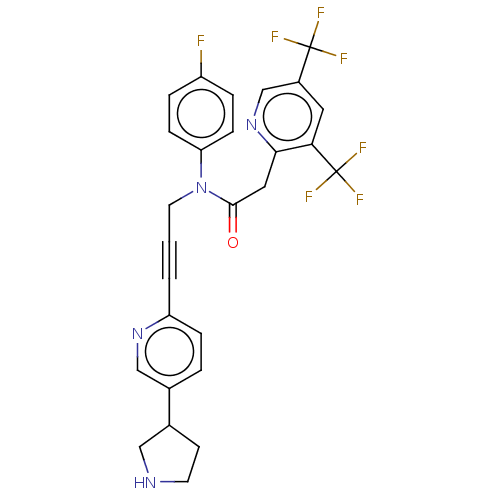
(CHEMBL5174509)Show SMILES Fc1ccc(cc1)N(CC#Cc1ccc(cn1)C1CCNC1)C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591026
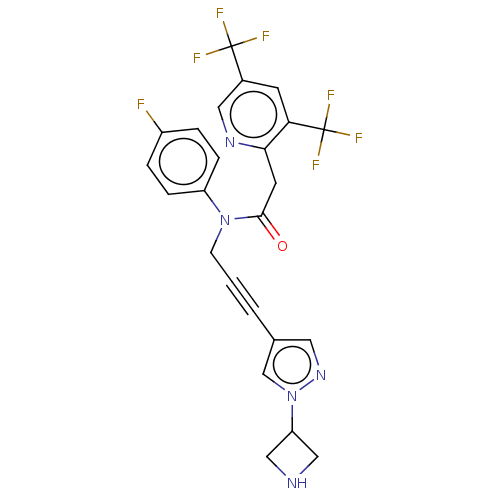
(CHEMBL5197173)Show SMILES Fc1ccc(cc1)N(CC#Cc1cnn(c1)C1CNC1)C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM290889

(1-[2-fluoro-4- (tetrahydro-2H- pyran-4-yl)phenyl]-...)Show SMILES COc1cn(nc(-c2ccnn2-c2ccccc2)c1=O)-c1ccc(cc1F)C1CCOCC1 Show InChI InChI=1S/C25H23FN4O3/c1-32-23-16-29(21-8-7-18(15-20(21)26)17-10-13-33-14-11-17)28-24(25(23)31)22-9-12-27-30(22)19-5-3-2-4-6-19/h2-9,12,15-17H,10-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591015
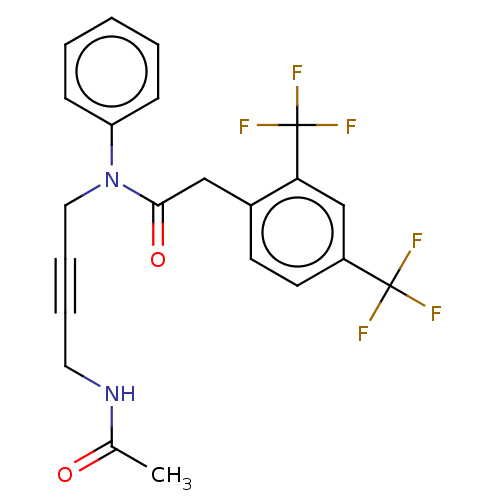
(CHEMBL5170373)Show SMILES CC(=O)NCC#CCN(C(=O)Cc1ccc(cc1C(F)(F)F)C(F)(F)F)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591028
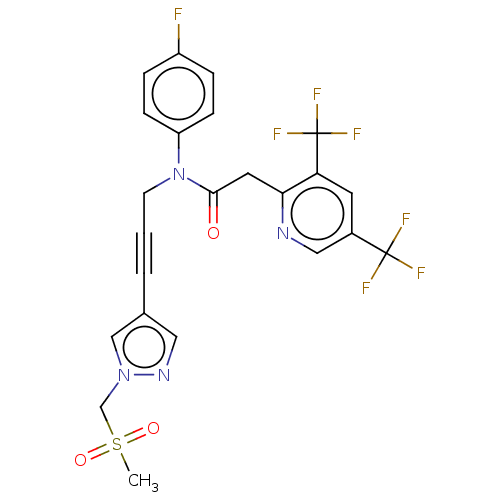
(CHEMBL5194637)Show SMILES CS(=O)(=O)Cn1cc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591027
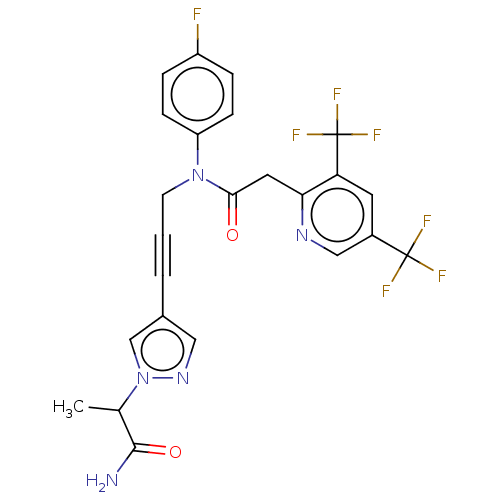
(CHEMBL5197021)Show SMILES CC(C(N)=O)n1cc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448086
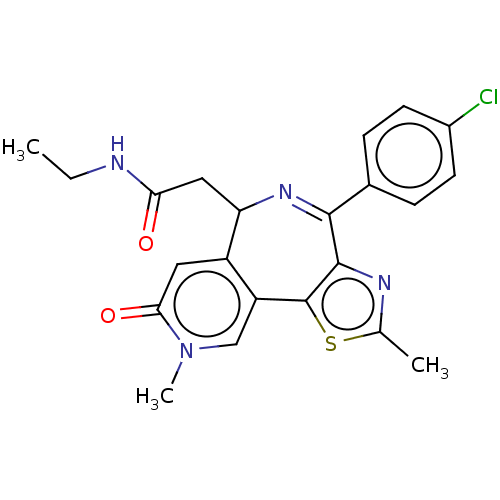
(US10689395, Compound m | US11267820, Compound m)Show SMILES CCNC(=O)CC1N=C(c2nc(C)sc2-c2cn(C)c(=O)cc12)c1ccc(Cl)cc1 |c:7| Show InChI InChI=1S/C22H21ClN4O2S/c1-4-24-18(28)10-17-15-9-19(29)27(3)11-16(15)22-21(25-12(2)30-22)20(26-17)13-5-7-14(23)8-6-13/h5-9,11,17H,4,10H2,1-3H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H1X |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448086

(US10689395, Compound m | US11267820, Compound m)Show SMILES CCNC(=O)CC1N=C(c2nc(C)sc2-c2cn(C)c(=O)cc12)c1ccc(Cl)cc1 |c:7| Show InChI InChI=1S/C22H21ClN4O2S/c1-4-24-18(28)10-17-15-9-19(29)27(3)11-16(15)22-21(25-12(2)30-22)20(26-17)13-5-7-14(23)8-6-13/h5-9,11,17H,4,10H2,1-3H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED
US Patent
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
US Patent US10689395 (2020)
BindingDB Entry DOI: 10.7270/Q21J9DTK |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448094

(US10689395, Compound ll | US11267820, Compound ll)Show SMILES CCNC(=O)CC1OC(c2c(cnn2C)-c2cn(C)c(=O)cc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H23ClN4O3/c1-4-24-19(28)10-18-15-9-20(29)26(2)12-17(15)16-11-25-27(3)21(16)22(30-18)13-5-7-14(23)8-6-13/h5-9,11-12,18,22H,4,10H2,1-3H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H1X |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448094

(US10689395, Compound ll | US11267820, Compound ll)Show SMILES CCNC(=O)CC1OC(c2c(cnn2C)-c2cn(C)c(=O)cc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H23ClN4O3/c1-4-24-19(28)10-18-15-9-20(29)26(2)12-17(15)16-11-25-27(3)21(16)22(30-18)13-5-7-14(23)8-6-13/h5-9,11-12,18,22H,4,10H2,1-3H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED
US Patent
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
US Patent US10689395 (2020)
BindingDB Entry DOI: 10.7270/Q21J9DTK |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448091
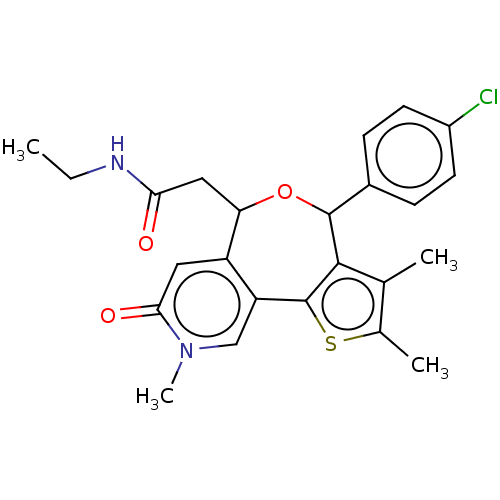
(US10689395, Compound bb | US11267820, Compound bb)Show SMILES CCNC(=O)CC1OC(c2c(C)c(C)sc2-c2cn(C)c(=O)cc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H25ClN2O3S/c1-5-26-20(28)11-19-17-10-21(29)27(4)12-18(17)24-22(13(2)14(3)31-24)23(30-19)15-6-8-16(25)9-7-15/h6-10,12,19,23H,5,11H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H1X |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448091

(US10689395, Compound bb | US11267820, Compound bb)Show SMILES CCNC(=O)CC1OC(c2c(C)c(C)sc2-c2cn(C)c(=O)cc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H25ClN2O3S/c1-5-26-20(28)11-19-17-10-21(29)27(4)12-18(17)24-22(13(2)14(3)31-24)23(30-19)15-6-8-16(25)9-7-15/h6-10,12,19,23H,5,11H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED
US Patent
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
US Patent US10689395 (2020)
BindingDB Entry DOI: 10.7270/Q21J9DTK |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591019
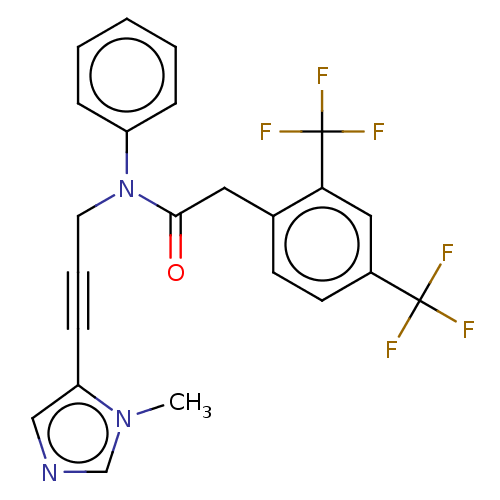
(CHEMBL5187337)Show SMILES Cn1cncc1C#CCN(C(=O)Cc1ccc(cc1C(F)(F)F)C(F)(F)F)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591024

(CHEMBL5188048)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2cccnn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50615563

(CHEMBL5273665)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4cn(-c5ccccc5C(F)(F)F)c5ccccc5c4=O)cc3F)ccnc2cc1OCCCN1CCCCC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591033
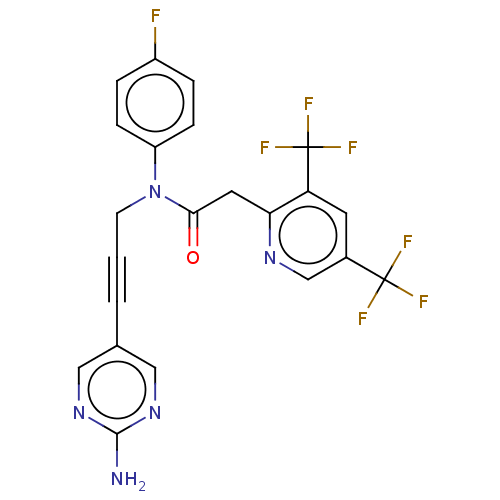
(CHEMBL5181369)Show SMILES Nc1ncc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591029
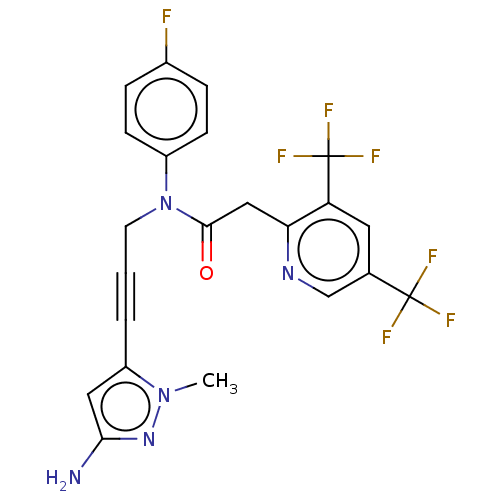
(CHEMBL5174376)Show SMILES Cn1nc(N)cc1C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50608970
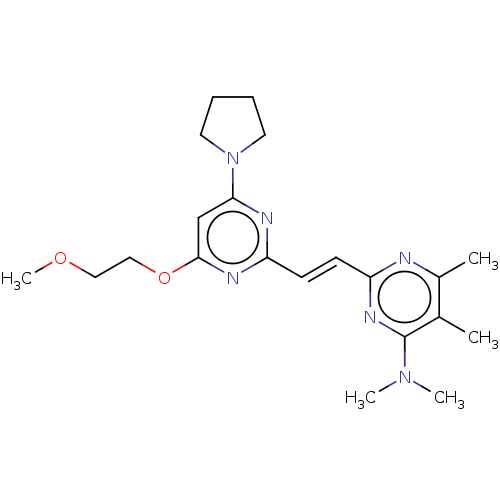
(CHEMBL5284754)Show SMILES COCCOc1cc(nc(\C=C\c2nc(C)c(C)c(n2)N(C)C)n1)N1CCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM290889

(1-[2-fluoro-4- (tetrahydro-2H- pyran-4-yl)phenyl]-...)Show SMILES COc1cn(nc(-c2ccnn2-c2ccccc2)c1=O)-c1ccc(cc1F)C1CCOCC1 Show InChI InChI=1S/C25H23FN4O3/c1-32-23-16-29(21-8-7-18(15-20(21)26)17-10-13-33-14-11-17)28-24(25(23)31)22-9-12-27-30(22)19-5-3-2-4-6-19/h2-9,12,15-17H,10-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365964
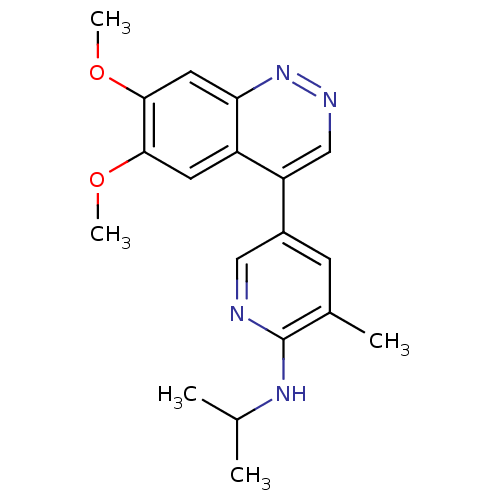
(CHEMBL1956235 | CHEMBL2070530)Show InChI InChI=1S/C19H22N4O2/c1-11(2)22-19-12(3)6-13(9-20-19)15-10-21-23-16-8-18(25-5)17(24-4)7-14(15)16/h6-11H,1-5H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448092

(US10689395, Compound ee | US11267820, Compound ee)Show SMILES Cc1sc-2c(C(OC(CC(N)=O)c3cc(=O)n(C)cc-23)c2ccc(Cl)cc2)c1C Show InChI InChI=1S/C22H21ClN2O3S/c1-11-12(2)29-22-16-10-25(3)19(27)8-15(16)17(9-18(24)26)28-21(20(11)22)13-4-6-14(23)7-5-13/h4-8,10,17,21H,9H2,1-3H3,(H2,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H1X |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448088
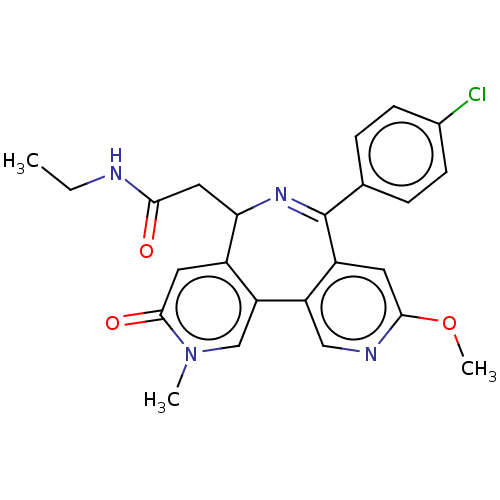
(US10689395, Compound t | US11267820, Compound t)Show SMILES CCNC(=O)CC1N=C(c2ccc(Cl)cc2)c2cc(OC)ncc2-c2cn(C)c(=O)cc12 |t:7| Show InChI InChI=1S/C24H23ClN4O3/c1-4-26-21(30)11-20-16-10-23(31)29(2)13-19(16)18-12-27-22(32-3)9-17(18)24(28-20)14-5-7-15(25)8-6-14/h5-10,12-13,20H,4,11H2,1-3H3,(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H1X |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448075
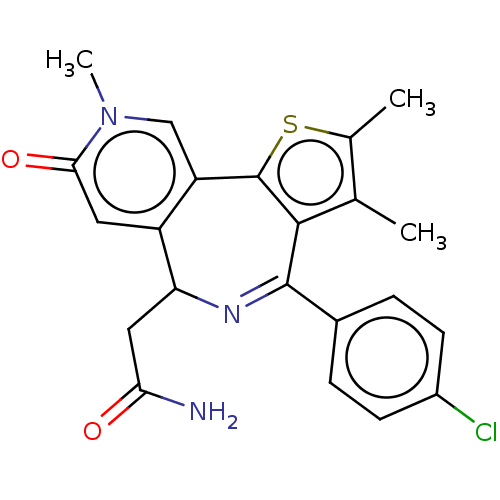
(US10689395, Compound e | US11267820, Compound e)Show SMILES Cc1sc-2c(c1C)C(=NC(CC(N)=O)c1cc(=O)n(C)cc-21)c1ccc(Cl)cc1 |c:8| Show InChI InChI=1S/C22H20ClN3O2S/c1-11-12(2)29-22-16-10-26(3)19(28)8-15(16)17(9-18(24)27)25-21(20(11)22)13-4-6-14(23)7-5-13/h4-8,10,17H,9H2,1-3H3,(H2,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H1X |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50520336
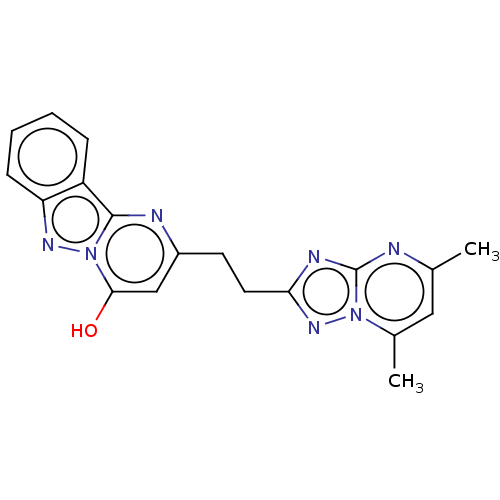
(CHEMBL4470042)Show SMILES Cc1cc(C)n2nc(CCc3cc(O)n4nc5ccccc5c4n3)nc2n1 Show InChI InChI=1S/C19H17N7O/c1-11-9-12(2)25-19(20-11)22-16(24-25)8-7-13-10-17(27)26-18(21-13)14-5-3-4-6-15(14)23-26/h3-6,9-10,27H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448092

(US10689395, Compound ee | US11267820, Compound ee)Show SMILES Cc1sc-2c(C(OC(CC(N)=O)c3cc(=O)n(C)cc-23)c2ccc(Cl)cc2)c1C Show InChI InChI=1S/C22H21ClN2O3S/c1-11-12(2)29-22-16-10-25(3)19(27)8-15(16)17(9-18(24)26)28-21(20(11)22)13-4-6-14(23)7-5-13/h4-8,10,17,21H,9H2,1-3H3,(H2,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED
US Patent
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
US Patent US10689395 (2020)
BindingDB Entry DOI: 10.7270/Q21J9DTK |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448088

(US10689395, Compound t | US11267820, Compound t)Show SMILES CCNC(=O)CC1N=C(c2ccc(Cl)cc2)c2cc(OC)ncc2-c2cn(C)c(=O)cc12 |t:7| Show InChI InChI=1S/C24H23ClN4O3/c1-4-26-21(30)11-20-16-10-23(31)29(2)13-19(16)18-12-27-22(32-3)9-17(18)24(28-20)14-5-7-15(25)8-6-14/h5-10,12-13,20H,4,11H2,1-3H3,(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED
US Patent
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
US Patent US10689395 (2020)
BindingDB Entry DOI: 10.7270/Q21J9DTK |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM448075

(US10689395, Compound e | US11267820, Compound e)Show SMILES Cc1sc-2c(c1C)C(=NC(CC(N)=O)c1cc(=O)n(C)cc-21)c1ccc(Cl)cc1 |c:8| Show InChI InChI=1S/C22H20ClN3O2S/c1-11-12(2)29-22-16-10-26(3)19(28)8-15(16)17(9-18(24)27)25-21(20(11)22)13-4-6-14(23)7-5-13/h4-8,10,17H,9H2,1-3H3,(H2,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
JUBILANT BIOSYS LIMITED
US Patent
| Assay Description
Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c... |
US Patent US10689395 (2020)
BindingDB Entry DOI: 10.7270/Q21J9DTK |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591032
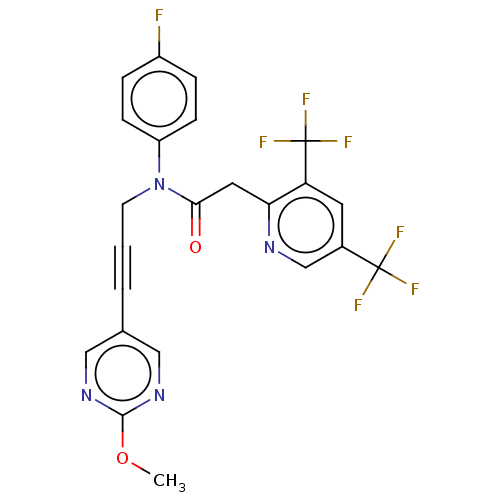
(CHEMBL5200250)Show SMILES COc1ncc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50608294
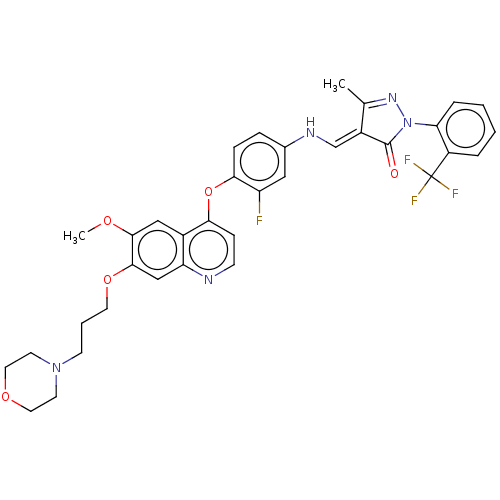
(CHEMBL3949465)Show SMILES COc1cc2c(Oc3ccc(N\C=C4\C(C)=NN(C4=O)c4ccccc4C(F)(F)F)cc3F)ccnc2cc1OCCCN1CCOCC1 |c:15| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data