 Found 236 hits with Last Name = 'mullins' and Initial = 'pb'
Found 236 hits with Last Name = 'mullins' and Initial = 'pb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM50078668

(3-(4-Phenylsulfanyl-benzenesulfonyl)-cyclohexaneth...)Show InChI InChI=1S/C18H20O2S3/c19-23(20,18-8-4-5-14(21)13-18)17-11-9-16(10-12-17)22-15-6-2-1-3-7-15/h1-3,6-7,9-12,14,18,21H,4-5,8,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50076589

(3-(4-Phenoxy-benzenesulfonyl)-propane-1-thiol | CH...)Show InChI InChI=1S/C15H16O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-3,5-10,19H,4,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196751
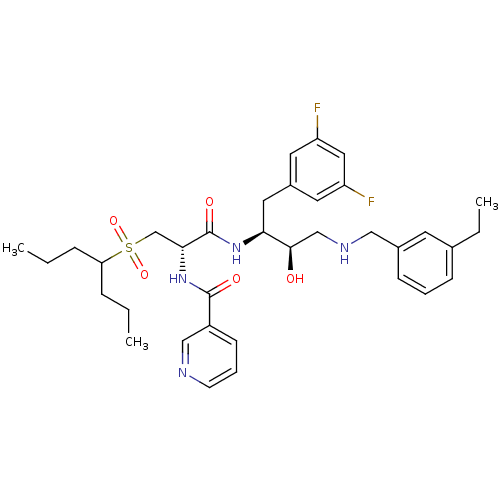
(CHEMBL231862 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1cccnc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C35H46F2N4O5S/c1-4-9-30(10-5-2)47(45,46)23-32(41-34(43)27-13-8-14-38-21-27)35(44)40-31(18-26-16-28(36)19-29(37)17-26)33(42)22-39-20-25-12-7-11-24(6-3)15-25/h7-8,11-17,19,21,30-33,39,42H,4-6,9-10,18,20,22-23H2,1-3H3,(H,40,44)(H,41,43)/t31-,32+,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50078667

(3-(4-Phenoxy-benzenesulfonyl)-cyclohexanethiol | C...)Show InChI InChI=1S/C18H20O3S2/c19-23(20,18-8-4-7-16(22)13-18)17-11-9-15(10-12-17)21-14-5-2-1-3-6-14/h1-3,5-6,9-12,16,18,22H,4,7-8,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574409
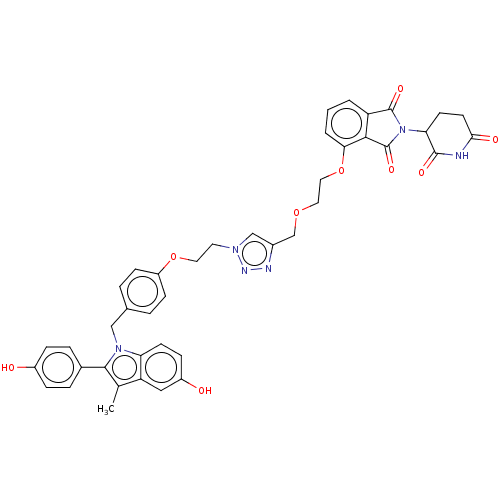
(CHEMBL4873534)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCn3cc(COCCOc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)nn3)cc2)c2ccc(O)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196747
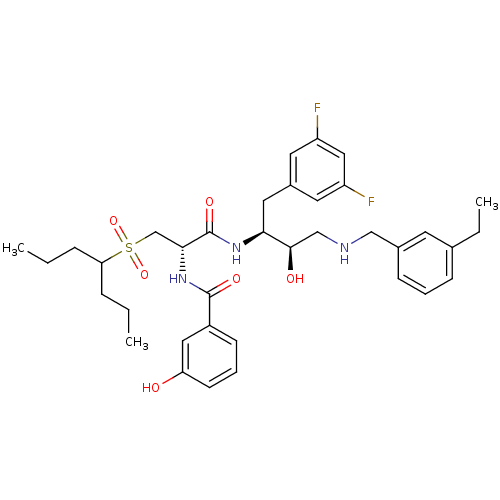
(CHEMBL266566 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1cccc(O)c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C36H47F2N3O6S/c1-4-9-31(10-5-2)48(46,47)23-33(41-35(44)27-13-8-14-30(42)19-27)36(45)40-32(18-26-16-28(37)20-29(38)17-26)34(43)22-39-21-25-12-7-11-24(6-3)15-25/h7-8,11-17,19-20,31-34,39,42-43H,4-6,9-10,18,21-23H2,1-3H3,(H,40,45)(H,41,44)/t32-,33+,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196755

(CHEMBL266567 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1cccnc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C32H40F2N4O5S/c1-3-5-12-44(42,43)21-29(38-31(40)25-10-7-11-35-19-25)32(41)37-28(16-24-14-26(33)17-27(34)15-24)30(39)20-36-18-23-9-6-8-22(4-2)13-23/h6-11,13-15,17,19,28-30,36,39H,3-5,12,16,18,20-21H2,1-2H3,(H,37,41)(H,38,40)/t28-,29+,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196746
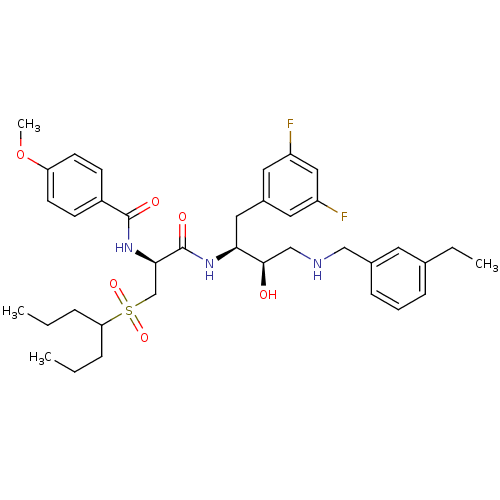
(CHEMBL231861 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1ccc(OC)cc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C37H49F2N3O6S/c1-5-9-32(10-6-2)49(46,47)24-34(42-36(44)28-13-15-31(48-4)16-14-28)37(45)41-33(20-27-18-29(38)21-30(39)19-27)35(43)23-40-22-26-12-8-11-25(7-3)17-26/h8,11-19,21,32-35,40,43H,5-7,9-10,20,22-24H2,1-4H3,(H,41,45)(H,42,44)/t33-,34+,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50076593
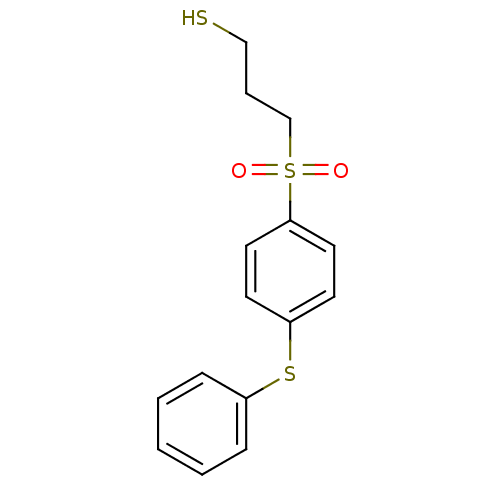
(3-(4-Phenylsulfanyl-benzenesulfonyl)-propane-1-thi...)Show InChI InChI=1S/C15H16O2S3/c16-20(17,12-4-11-18)15-9-7-14(8-10-15)19-13-5-2-1-3-6-13/h1-3,5-10,18H,4,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574409

(CHEMBL4873534)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCn3cc(COCCOc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)nn3)cc2)c2ccc(O)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha S463P mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196736

(CHEMBL231863 | benzyl (S)-1-((2S,3R)-4-(3-ethylben...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C37H49F2N3O6S/c1-4-11-32(12-5-2)49(46,47)25-34(42-37(45)48-24-27-13-8-7-9-14-27)36(44)41-33(20-29-18-30(38)21-31(39)19-29)35(43)23-40-22-28-16-10-15-26(6-3)17-28/h7-10,13-19,21,32-35,40,43H,4-6,11-12,20,22-25H2,1-3H3,(H,41,44)(H,42,45)/t33-,34+,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196745

(CHEMBL414145 | methyl (S)-1-((2S,3R)-4-(3-ethylben...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)OC)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C31H45F2N3O6S/c1-5-9-26(10-6-2)43(40,41)20-28(36-31(39)42-4)30(38)35-27(16-23-14-24(32)17-25(33)15-23)29(37)19-34-18-22-12-8-11-21(7-3)13-22/h8,11-15,17,26-29,34,37H,5-7,9-10,16,18-20H2,1-4H3,(H,35,38)(H,36,39)/t27-,28+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196749
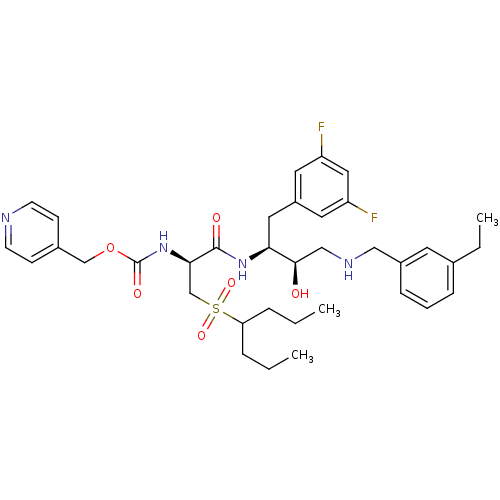
(CHEMBL394270 | pyridin-4-ylmethyl (S)-1-((2S,3R)-4...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)OCc1ccncc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C36H48F2N4O6S/c1-4-8-31(9-5-2)49(46,47)24-33(42-36(45)48-23-26-12-14-39-15-13-26)35(44)41-32(19-28-17-29(37)20-30(38)18-28)34(43)22-40-21-27-11-7-10-25(6-3)16-27/h7,10-18,20,31-34,40,43H,4-6,8-9,19,21-24H2,1-3H3,(H,41,44)(H,42,45)/t32-,33+,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574409

(CHEMBL4873534)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCn3cc(COCCOc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)nn3)cc2)c2ccc(O)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha Y537S mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196757
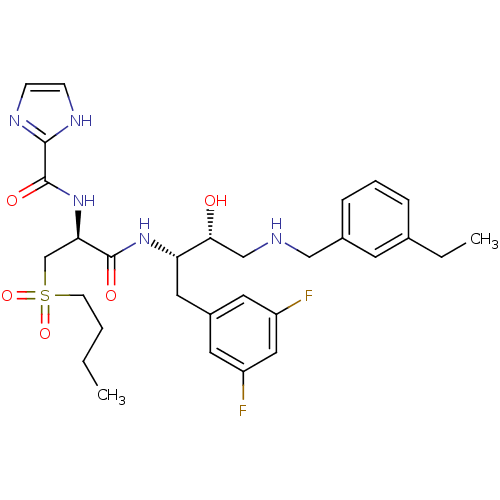
(CHEMBL391144 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1ncc[nH]1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C30H39F2N5O5S/c1-3-5-11-43(41,42)19-26(37-30(40)28-34-9-10-35-28)29(39)36-25(15-22-13-23(31)16-24(32)14-22)27(38)18-33-17-21-8-6-7-20(4-2)12-21/h6-10,12-14,16,25-27,33,38H,3-5,11,15,17-19H2,1-2H3,(H,34,35)(H,36,39)(H,37,40)/t25-,26+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50076589

(3-(4-Phenoxy-benzenesulfonyl)-propane-1-thiol | CH...)Show InChI InChI=1S/C15H16O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-3,5-10,19H,4,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-8 (hMMP-8) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196733

(CHEMBL414776 | pyridin-3-ylmethyl (S)-1-((2S,3R)-4...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)OCc1cccnc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C36H48F2N4O6S/c1-4-9-31(10-5-2)49(46,47)24-33(42-36(45)48-23-27-13-8-14-39-21-27)35(44)41-32(18-28-16-29(37)19-30(38)17-28)34(43)22-40-20-26-12-7-11-25(6-3)15-26/h7-8,11-17,19,21,31-34,40,43H,4-6,9-10,18,20,22-24H2,1-3H3,(H,41,44)(H,42,45)/t32-,33+,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196744
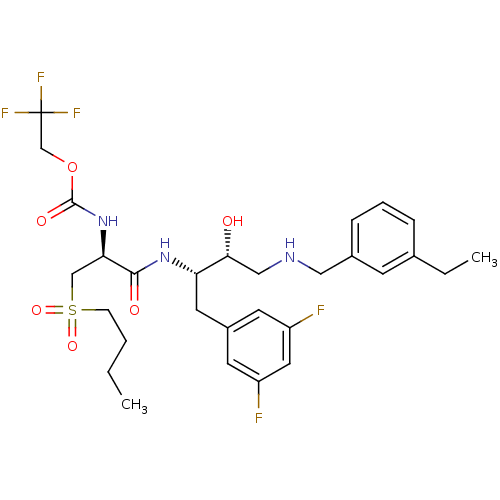
(2,2,2-trifluoroethyl (S)-1-((2S,3R)-4-(3-ethylbenz...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)OCC(F)(F)F)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C29H38F5N3O6S/c1-3-5-9-44(41,42)17-25(37-28(40)43-18-29(32,33)34)27(39)36-24(13-21-11-22(30)14-23(31)12-21)26(38)16-35-15-20-8-6-7-19(4-2)10-20/h6-8,10-12,14,24-26,35,38H,3-5,9,13,15-18H2,1-2H3,(H,36,39)(H,37,40)/t24-,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196754
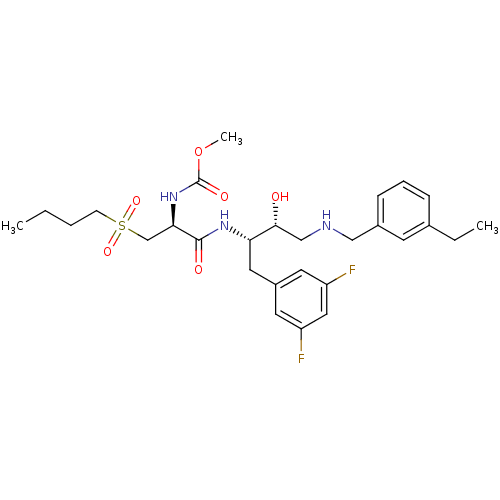
(CHEMBL393733 | methyl (S)-1-((2S,3R)-4-(3-ethylben...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)OC)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C28H39F2N3O6S/c1-4-6-10-40(37,38)18-25(33-28(36)39-3)27(35)32-24(14-21-12-22(29)15-23(30)13-21)26(34)17-31-16-20-9-7-8-19(5-2)11-20/h7-9,11-13,15,24-26,31,34H,4-6,10,14,16-18H2,1-3H3,(H,32,35)(H,33,36)/t24-,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196752

(CHEMBL412852 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1ccc(C)cc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C37H49F2N3O5S/c1-5-9-32(10-6-2)48(46,47)24-34(42-36(44)29-15-13-25(4)14-16-29)37(45)41-33(20-28-18-30(38)21-31(39)19-28)35(43)23-40-22-27-12-8-11-26(7-3)17-27/h8,11-19,21,32-35,40,43H,5-7,9-10,20,22-24H2,1-4H3,(H,41,45)(H,42,44)/t33-,34+,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196740
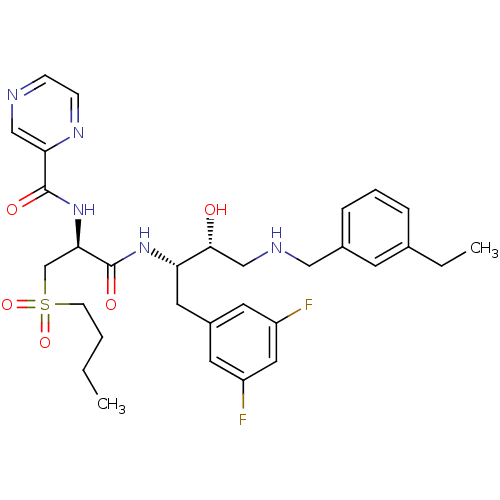
(CHEMBL409875 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1cnccn1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C31H39F2N5O5S/c1-3-5-11-44(42,43)20-28(38-30(40)27-18-34-9-10-36-27)31(41)37-26(15-23-13-24(32)16-25(33)14-23)29(39)19-35-17-22-8-6-7-21(4-2)12-22/h6-10,12-14,16,18,26,28-29,35,39H,3-5,11,15,17,19-20H2,1-2H3,(H,37,41)(H,38,40)/t26-,28+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196734

(CHEMBL409947 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1cc(C)n[nH]1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C31H41F2N5O5S/c1-4-6-10-44(42,43)19-28(36-30(40)27-11-20(3)37-38-27)31(41)35-26(15-23-13-24(32)16-25(33)14-23)29(39)18-34-17-22-9-7-8-21(5-2)12-22/h7-9,11-14,16,26,28-29,34,39H,4-6,10,15,17-19H2,1-3H3,(H,35,41)(H,36,40)(H,37,38)/t26-,28+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574409

(CHEMBL4873534)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCn3cc(COCCOc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)nn3)cc2)c2ccc(O)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha D538G mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196739
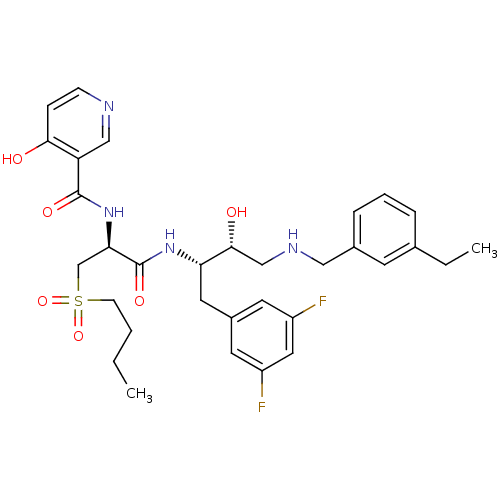
(CHEMBL266531 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1cnccc1O)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C32H40F2N4O6S/c1-3-5-11-45(43,44)20-28(38-31(41)26-18-35-10-9-29(26)39)32(42)37-27(15-23-13-24(33)16-25(34)14-23)30(40)19-36-17-22-8-6-7-21(4-2)12-22/h6-10,12-14,16,18,27-28,30,36,40H,3-5,11,15,17,19-20H2,1-2H3,(H,35,39)(H,37,42)(H,38,41)/t27-,28+,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196750
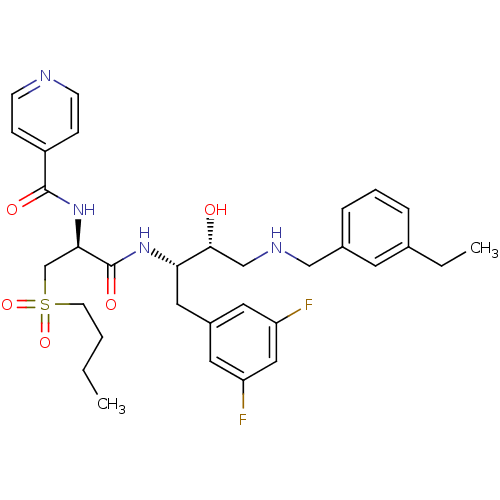
(CHEMBL394271 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1ccncc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C32H40F2N4O5S/c1-3-5-13-44(42,43)21-29(38-31(40)25-9-11-35-12-10-25)32(41)37-28(17-24-15-26(33)18-27(34)16-24)30(39)20-36-19-23-8-6-7-22(4-2)14-23/h6-12,14-16,18,28-30,36,39H,3-5,13,17,19-21H2,1-2H3,(H,37,41)(H,38,40)/t28-,29+,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM15797
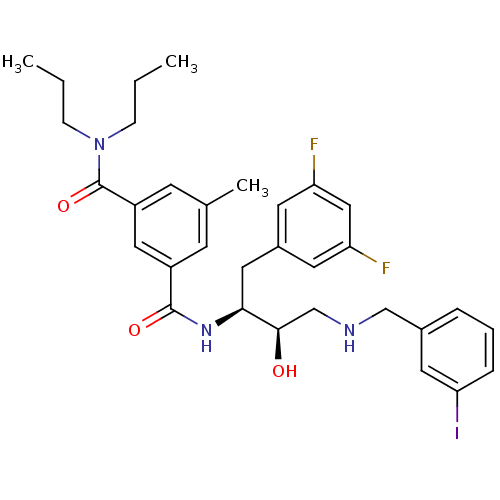
((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(3-i...)Show SMILES CCCN(CCC)C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(I)c1 |r| Show InChI InChI=1S/C32H38F2IN3O3/c1-4-9-38(10-5-2)32(41)25-12-21(3)11-24(17-25)31(40)37-29(16-23-13-26(33)18-27(34)14-23)30(39)20-36-19-22-7-6-8-28(35)15-22/h6-8,11-15,17-18,29-30,36,39H,4-5,9-10,16,19-20H2,1-3H3,(H,37,40)/t29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE |
Bioorg Med Chem Lett 17: 73-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.092
BindingDB Entry DOI: 10.7270/Q2FX793P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196753
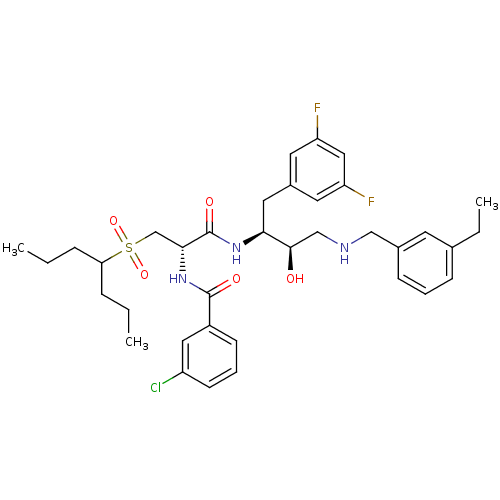
(CHEMBL266565 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1cccc(Cl)c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C36H46ClF2N3O5S/c1-4-9-31(10-5-2)48(46,47)23-33(42-35(44)27-13-8-14-28(37)19-27)36(45)41-32(18-26-16-29(38)20-30(39)17-26)34(43)22-40-21-25-12-7-11-24(6-3)15-25/h7-8,11-17,19-20,31-34,40,43H,4-6,9-10,18,21-23H2,1-3H3,(H,41,45)(H,42,44)/t32-,33+,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574413
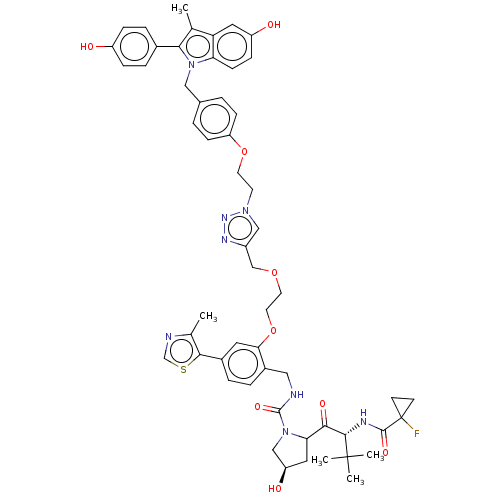
(CHEMBL4873959)Show SMILES Cc1ncsc1-c1ccc(CNC(=O)N2C[C@H](O)CC2C(=O)[C@H](NC(=O)C2(F)CC2)C(C)(C)C)c(OCCOCc2cn(CCOc3ccc(Cn4c(c(C)c5cc(O)ccc45)-c4ccc(O)cc4)cc3)nn2)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574412
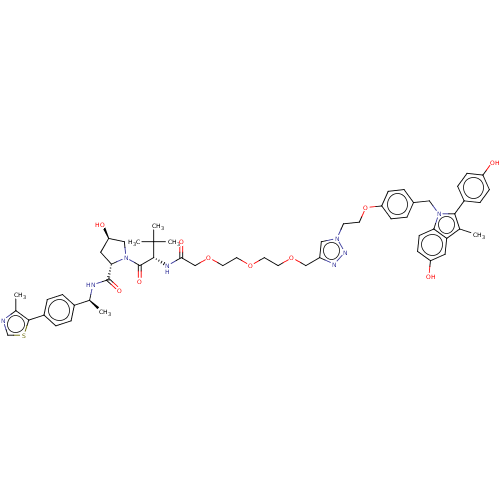
(CHEMBL4856006)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)COCCOCCOCc1cn(CCOc2ccc(Cn3c(c(C)c4cc(O)ccc34)-c3ccc(O)cc3)cc2)nn1)C(C)(C)C)c1ccc(cc1)-c1scnc1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha S463P mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50096851

(CHEMBL3580774)Show SMILES C[C@@H](COc1ccc(F)cc1[C@@H](C)C(F)(F)F)N1CCn2c(ccc(-n3cnc(C)c3)c2=O)C1=O |r| Show InChI InChI=1S/C24H24F4N4O3/c1-14-11-30(13-29-14)19-5-6-20-23(34)31(8-9-32(20)22(19)33)15(2)12-35-21-7-4-17(25)10-18(21)16(3)24(26,27)28/h4-7,10-11,13,15-16H,8-9,12H2,1-3H3/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Modulation of human gamma secretase overexpressed in CHO cells co-expressing wild type human APP assessed as inhibition of amyloid beta-42 production... |
ACS Med Chem Lett 6: 596-601 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00070
BindingDB Entry DOI: 10.7270/Q26975BS |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574401

(CHEMBL4855559) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha D538G mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574414
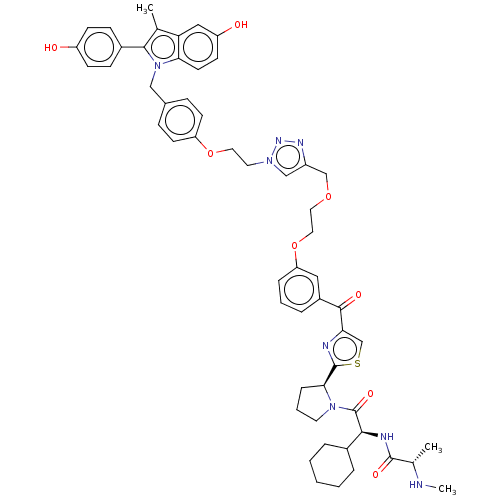
(CHEMBL4850845)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1cccc(OCCOCc2cn(CCOc3ccc(Cn4c(c(C)c5cc(O)ccc45)-c4ccc(O)cc4)cc3)nn2)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574406

(CHEMBL4851230)Show SMILES Oc1cccc(CN(CC2(CCCC2)c2ccccc2F)c2ccc(OCCN3CCCCC3)cc2)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50078667

(3-(4-Phenoxy-benzenesulfonyl)-cyclohexanethiol | C...)Show InChI InChI=1S/C18H20O3S2/c19-23(20,18-8-4-7-16(22)13-18)17-11-9-15(10-12-17)21-14-5-2-1-3-6-14/h1-3,5-6,9-12,16,18,22H,4,7-8,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-8 (hMMP-8) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196758

(2-cyanoethyl 1-((2S,3R)-4-(3-ethylbenzylamino)-1-(...)Show SMILES CCCCS(=O)(=O)CC(NC(=O)OCCC#N)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C30H40F2N4O6S/c1-3-5-12-43(40,41)20-27(36-30(39)42-11-7-10-33)29(38)35-26(16-23-14-24(31)17-25(32)15-23)28(37)19-34-18-22-9-6-8-21(4-2)13-22/h6,8-9,13-15,17,26-28,34,37H,3-5,7,11-12,16,18-20H2,1-2H3,(H,35,38)(H,36,39)/t26-,27?,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574401

(CHEMBL4855559) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha Y537S mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574410

(CHEMBL4851860)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)COCc1cn(CCOc2ccc(Cn3c(c(C)c4cc(O)ccc34)-c3ccc(O)cc3)cc2)nn1)C(C)(C)C)c1ccc(cc1)-c1scnc1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha Y537S mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574412

(CHEMBL4856006)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)COCCOCCOCc1cn(CCOc2ccc(Cn3c(c(C)c4cc(O)ccc34)-c3ccc(O)cc3)cc2)nn1)C(C)(C)C)c1ccc(cc1)-c1scnc1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha D538G mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196731

(CHEMBL231669 | N-{(S)-2-(butane-1-sulfonyl)-1-[(1S...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)C1CC1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C30H41F2N3O5S/c1-3-5-11-41(39,40)19-27(35-29(37)23-9-10-23)30(38)34-26(15-22-13-24(31)16-25(32)14-22)28(36)18-33-17-21-8-6-7-20(4-2)12-21/h6-8,12-14,16,23,26-28,33,36H,3-5,9-11,15,17-19H2,1-2H3,(H,34,38)(H,35,37)/t26-,27+,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196756
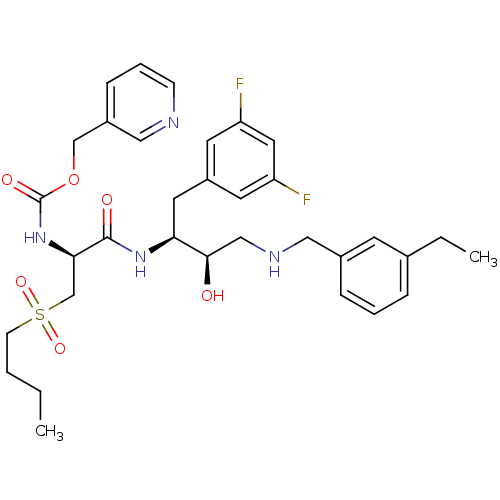
(CHEMBL409949 | pyridin-3-ylmethyl (S)-1-((2S,3R)-4...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)OCc1cccnc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C33H42F2N4O6S/c1-3-5-12-46(43,44)22-30(39-33(42)45-21-25-10-7-11-36-19-25)32(41)38-29(16-26-14-27(34)17-28(35)15-26)31(40)20-37-18-24-9-6-8-23(4-2)13-24/h6-11,13-15,17,19,29-31,37,40H,3-5,12,16,18,20-22H2,1-2H3,(H,38,41)(H,39,42)/t29-,30+,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196743

(CHEMBL409950 | pyridin-4-ylmethyl (S)-1-((2S,3R)-4...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)OCc1ccncc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C33H42F2N4O6S/c1-3-5-13-46(43,44)22-30(39-33(42)45-21-24-9-11-36-12-10-24)32(41)38-29(17-26-15-27(34)18-28(35)16-26)31(40)20-37-19-25-8-6-7-23(4-2)14-25/h6-12,14-16,18,29-31,37,40H,3-5,13,17,19-22H2,1-2H3,(H,38,41)(H,39,42)/t29-,30+,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574410

(CHEMBL4851860)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)COCc1cn(CCOc2ccc(Cn3c(c(C)c4cc(O)ccc34)-c3ccc(O)cc3)cc2)nn1)C(C)(C)C)c1ccc(cc1)-c1scnc1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196741

((S)-N-((2S,3R)-4-(3-ethylbenzylamino)-1-(3,5-diflu...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(C)=O)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C28H39F2N3O5S/c1-4-6-10-39(37,38)18-26(32-19(3)34)28(36)33-25(14-22-12-23(29)15-24(30)13-22)27(35)17-31-16-21-9-7-8-20(5-2)11-21/h7-9,11-13,15,25-27,31,35H,4-6,10,14,16-18H2,1-3H3,(H,32,34)(H,33,36)/t25-,26+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574413

(CHEMBL4873959)Show SMILES Cc1ncsc1-c1ccc(CNC(=O)N2C[C@H](O)CC2C(=O)[C@H](NC(=O)C2(F)CC2)C(C)(C)C)c(OCCOCc2cn(CCOc3ccc(Cn4c(c(C)c5cc(O)ccc45)-c4ccc(O)cc4)cc3)nn2)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha S463P mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574412

(CHEMBL4856006)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)COCCOCCOCc1cn(CCOc2ccc(Cn3c(c(C)c4cc(O)ccc34)-c3ccc(O)cc3)cc2)nn1)C(C)(C)C)c1ccc(cc1)-c1scnc1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha Y537S mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196737
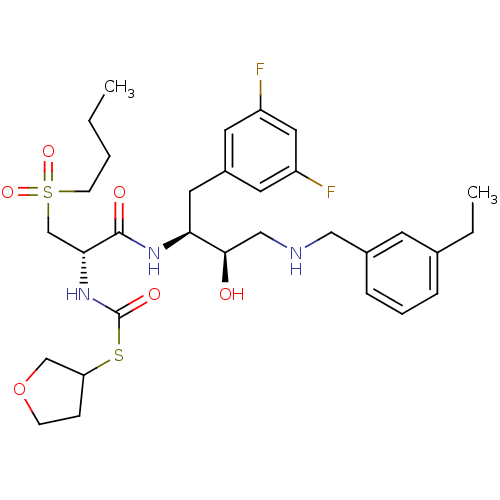
(CHEMBL409948 | S-tetrahydrofuran-3-yl (S)-1-((2S,3...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)SC1CCOC1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C31H43F2N3O6S2/c1-3-5-11-44(40,41)20-28(36-31(39)43-26-9-10-42-19-26)30(38)35-27(15-23-13-24(32)16-25(33)14-23)29(37)18-34-17-22-8-6-7-21(4-2)12-22/h6-8,12-14,16,26-29,34,37H,3-5,9-11,15,17-20H2,1-2H3,(H,35,38)(H,36,39)/t26?,27-,28+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196742

(CHEMBL393140 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1ccccc1C)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C37H49F2N3O5S/c1-5-11-31(12-6-2)48(46,47)24-34(42-36(44)32-16-9-8-13-25(32)4)37(45)41-33(20-28-18-29(38)21-30(39)19-28)35(43)23-40-22-27-15-10-14-26(7-3)17-27/h8-10,13-19,21,31,33-35,40,43H,5-7,11-12,20,22-24H2,1-4H3,(H,41,45)(H,42,44)/t33-,34+,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574401

(CHEMBL4855559) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574402

(CHEMBL4878045)Show SMILES Oc1cccc(CN(CC2(CCCC2)c2ccccc2F)c2ccccc2)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50096863

(CHEMBL3580766)Show SMILES Cc1cn(cn1)-c1ccc2C(=O)N(CCOc3ccccc3-c3cc(no3)C(F)(F)F)CCn2c1=O Show InChI InChI=1S/C24H20F3N5O4/c1-15-13-31(14-28-15)17-6-7-18-22(33)30(8-9-32(18)23(17)34)10-11-35-19-5-3-2-4-16(19)20-12-21(29-36-20)24(25,26)27/h2-7,12-14H,8-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Modulation of human gamma secretase overexpressed in CHO cells co-expressing wild type human APP assessed as inhibition of amyloid beta-42 production... |
ACS Med Chem Lett 6: 596-601 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00070
BindingDB Entry DOI: 10.7270/Q26975BS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data