 Found 682 hits with Last Name = 'timmermans' and Initial = 'pb'
Found 682 hits with Last Name = 'timmermans' and Initial = 'pb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor
(RABBIT) | BDBM50240609
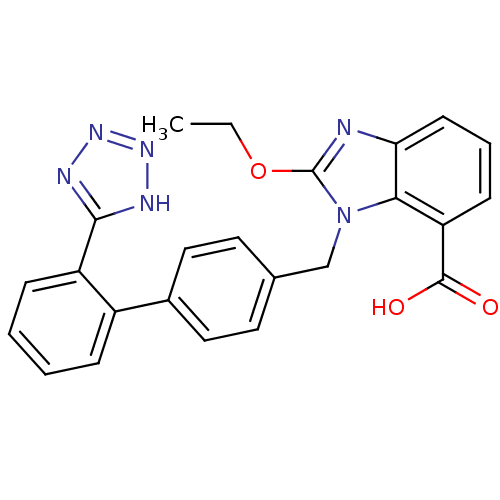
(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 in rat adrenal membrane |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50241491
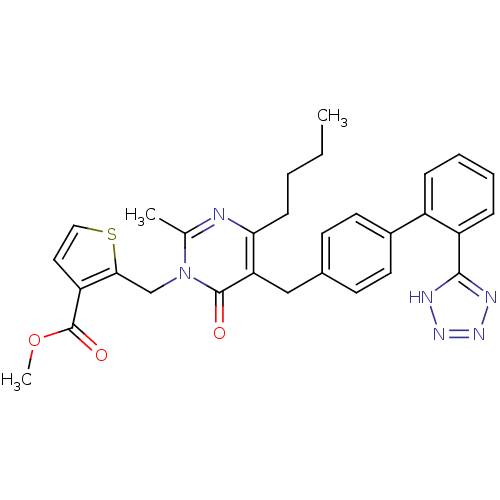
(2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCc1nc(C)n(Cc2sccc2C(=O)OC)c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H30N6O3S/c1-4-5-10-26-25(29(37)36(19(2)31-26)18-27-24(15-16-40-27)30(38)39-3)17-20-11-13-21(14-12-20)22-8-6-7-9-23(22)28-32-34-35-33-28/h6-9,11-16H,4-5,10,17-18H2,1-3H3,(H,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated towards Angiotensin II receptor, type 1 in rabbit aorta |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM30712
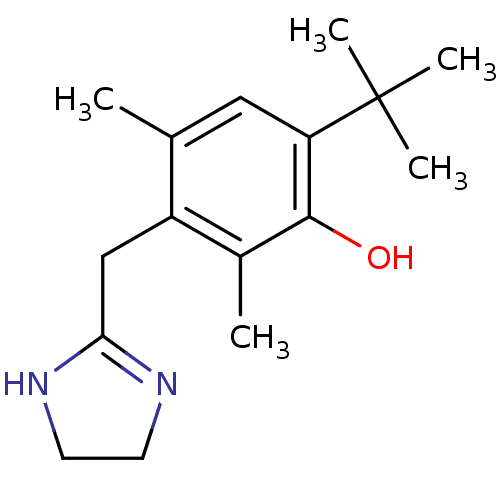
(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The ability to displace [3H]clonidine from the Alpha-2 adrenergic receptor was determined in rat brain membrane |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50027056
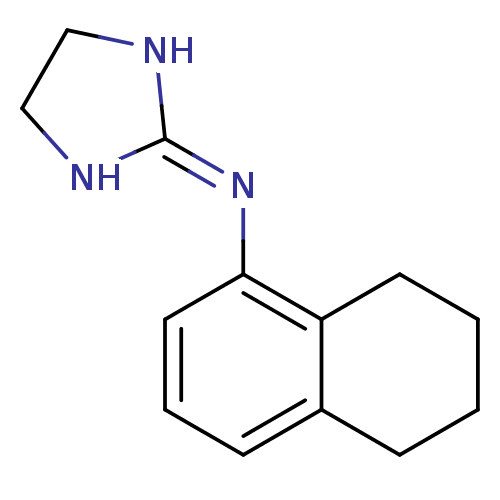
((4,5-Dihydro-1H-imidazol-2-yl)-(5,6,7,8-tetrahydro...)Show SMILES [#6]-1-[#6]-[#7]\[#6](-[#7]-1)=[#7]\c1cccc2-[#6]-[#6]-[#6]-[#6]-c12 Show InChI InChI=1S/C13H17N3/c1-2-6-11-10(4-1)5-3-7-12(11)16-13-14-8-9-15-13/h3,5,7H,1-2,4,6,8-9H2,(H2,14,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50016897
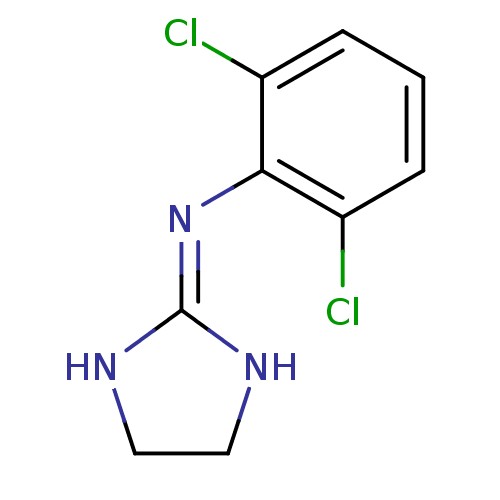
(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50001922
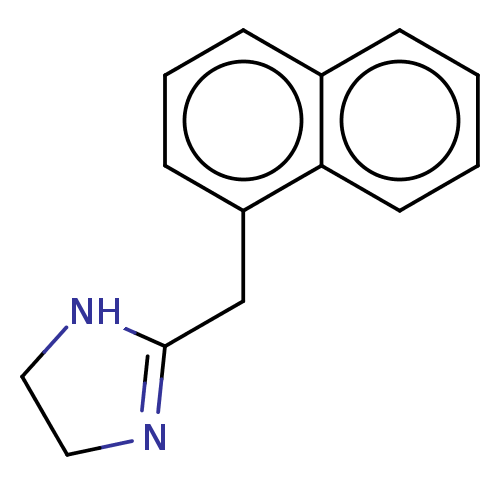
(2-Naphthalen-1-ylmethyl-4,5-dihydro-1H-imidazole; ...)Show InChI InChI=1S/C14H14N2/c1-2-7-13-11(4-1)5-3-6-12(13)10-14-15-8-9-16-14/h1-7H,8-10H2,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50223426
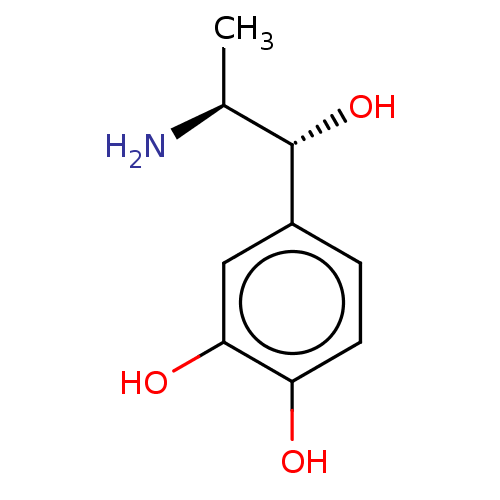
(CHEBI:10304 | Corbadrine | Levonordefrin | Neo-Cob...)Show SMILES [H][C@@](C)(N)[C@]([H])(O)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C9H13NO3/c1-5(10)9(13)6-2-3-7(11)8(12)4-6/h2-5,9,11-13H,10H2,1H3/t5-,9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50029051
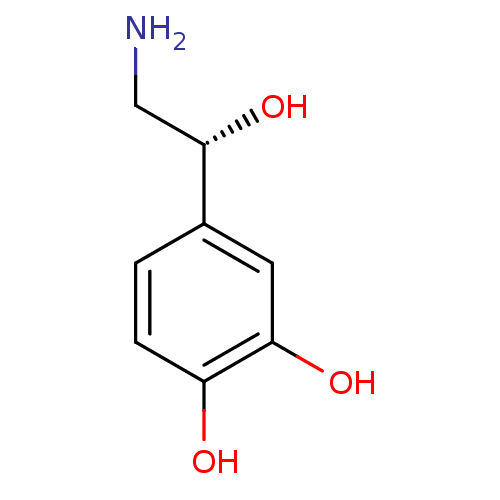
((-)-arterenol | (-)-noradrenaline | (-)-norepineph...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2/t8-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049210
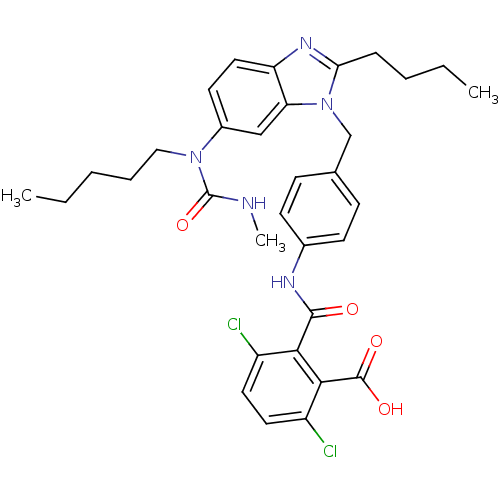
(CHEMBL348126 | N-{4-[2-Butyl-6-(3-methyl-1-pentyl-...)Show SMILES CCCCCN(C(=O)NC)c1ccc2nc(CCCC)n(Cc3ccc(NC(=O)c4c(Cl)ccc(Cl)c4C(O)=O)cc3)c2c1 Show InChI InChI=1S/C33H37Cl2N5O4/c1-4-6-8-18-39(33(44)36-3)23-14-17-26-27(19-23)40(28(38-26)9-7-5-2)20-21-10-12-22(13-11-21)37-31(41)29-24(34)15-16-25(35)30(29)32(42)43/h10-17,19H,4-9,18,20H2,1-3H3,(H,36,44)(H,37,41)(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Ki value was evaluated against Angiotensin II receptor, type 1 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM31046
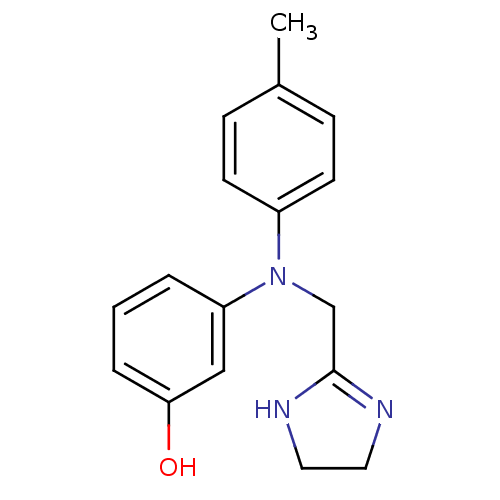
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50035429

(4'-[2-Butyl-6-(3-cyclohexyl-ureido)-benzoimidazol-...)Show SMILES CCCCc1nc2ccc(NC(=O)NC3CCCCC3)cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C32H36N4O3/c1-2-3-13-30-35-28-19-18-25(34-32(39)33-24-9-5-4-6-10-24)20-29(28)36(30)21-22-14-16-23(17-15-22)26-11-7-8-12-27(26)31(37)38/h7-8,11-12,14-20,24H,2-6,9-10,13,21H2,1H3,(H,37,38)(H2,33,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Ki value was evaluated against Angiotensin II receptor, type 1 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50017720
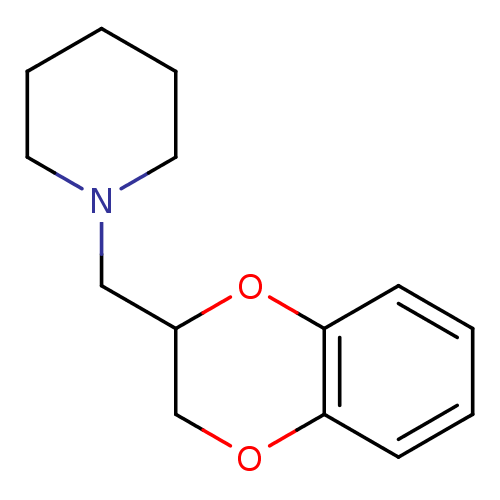
(1-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-piperi...)Show InChI InChI=1S/C14H19NO2/c1-4-8-15(9-5-1)10-12-11-16-13-6-2-3-7-14(13)17-12/h2-3,6-7,12H,1,4-5,8-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50026636

(17alpha-hydroxy-20alpha-yohimban-16beta-carboxylic...)Show SMILES COC(=O)[C@@H]1[C@@H](O)CC[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15+,17+,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM55436
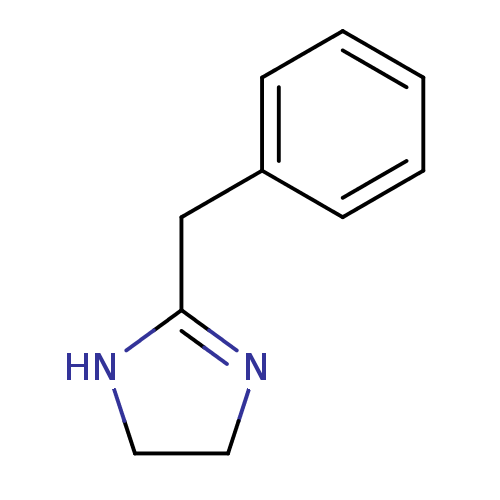
(2-(phenylmethyl)-4,5-dihydro-1H-imidazole;hydrochl...)Show InChI InChI=1S/C10H12N2/c1-2-4-9(5-3-1)8-10-11-6-7-12-10/h1-5H,6-8H2,(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM69602
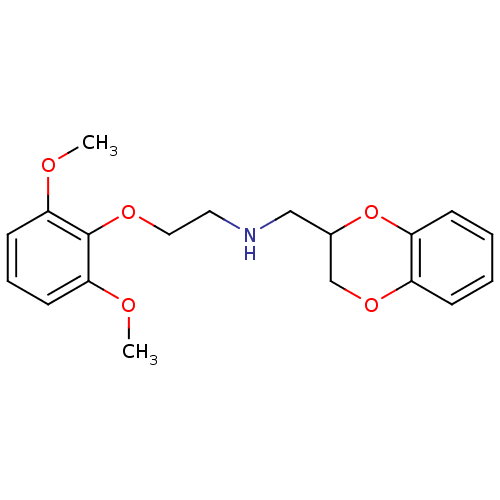
(2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...)Show InChI InChI=1S/C19H23NO5/c1-21-17-8-5-9-18(22-2)19(17)23-11-10-20-12-14-13-24-15-6-3-4-7-16(15)25-14/h3-9,14,20H,10-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50067212
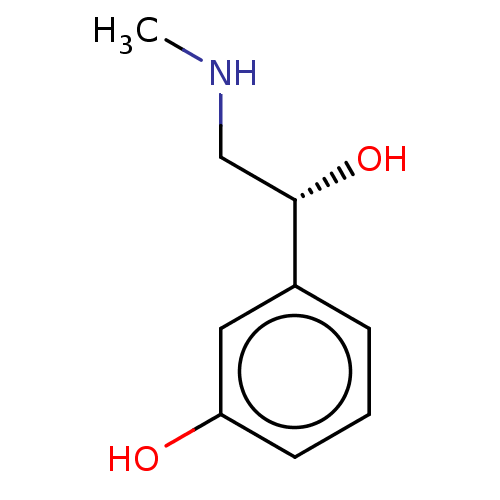
(CHEBI:8093 | Cyclomydril | Duo-Medihaler | Phenyle...)Show InChI InChI=1S/C9H13NO2/c1-10-6-9(12)7-3-2-4-8(11)5-7/h2-5,9-12H,6H2,1H3/t9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50035429

(4'-[2-Butyl-6-(3-cyclohexyl-ureido)-benzoimidazol-...)Show SMILES CCCCc1nc2ccc(NC(=O)NC3CCCCC3)cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C32H36N4O3/c1-2-3-13-30-35-28-19-18-25(34-32(39)33-24-9-5-4-6-10-24)20-29(28)36(30)21-22-14-16-23(17-15-22)26-11-7-8-12-27(26)31(37)38/h7-8,11-12,14-20,24H,2-6,9-10,13,21H2,1H3,(H,37,38)(H2,33,34,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Ki value was evaluated against Angiotensin II receptor, type 2 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049210

(CHEMBL348126 | N-{4-[2-Butyl-6-(3-methyl-1-pentyl-...)Show SMILES CCCCCN(C(=O)NC)c1ccc2nc(CCCC)n(Cc3ccc(NC(=O)c4c(Cl)ccc(Cl)c4C(O)=O)cc3)c2c1 Show InChI InChI=1S/C33H37Cl2N5O4/c1-4-6-8-18-39(33(44)36-3)23-14-17-26-27(19-23)40(28(38-26)9-7-5-2)20-21-10-12-22(13-11-21)37-31(41)29-24(34)15-16-25(35)30(29)32(42)43/h10-17,19H,4-9,18,20H2,1-3H3,(H,36,44)(H,37,41)(H,42,43) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Ki value was evaluated against Angiotensin II receptor, type 2 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]clonidine from Alpha-2 adrenergic receptor of rat brain membranes |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50027058
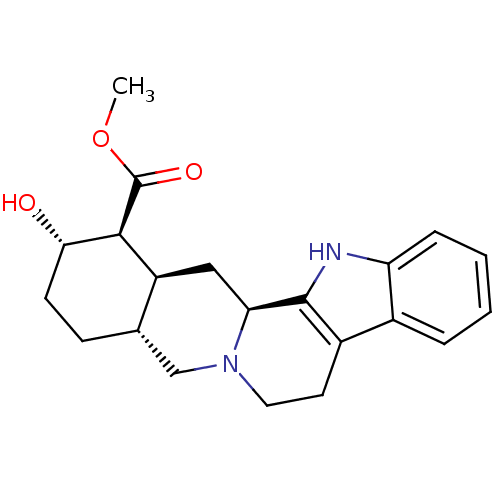
((1S,2S,4aR,13bS,14aS)-2-Hydroxy-1,2,3,4,4a,5,7,8,1...)Show SMILES COC(=O)[C@@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The ability to displace [3H]clonidine from the Alpha-2 adrenergic receptor was determined in rat brain membrane |
J Med Chem 25: 1389-401 (1982)
BindingDB Entry DOI: 10.7270/Q2251MCQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049199
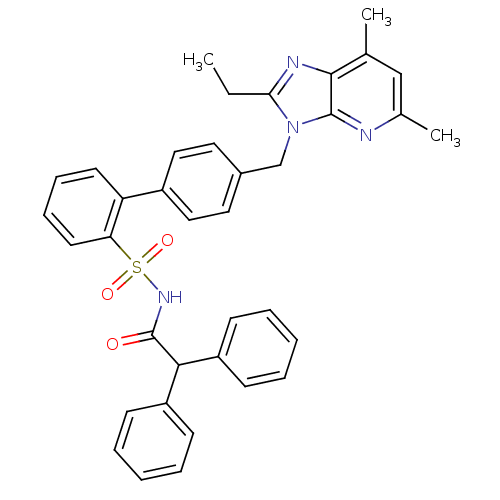
(4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C37H34N4O3S/c1-4-33-39-35-25(2)23-26(3)38-36(35)41(33)24-27-19-21-28(22-20-27)31-17-11-12-18-32(31)45(43,44)40-37(42)34(29-13-7-5-8-14-29)30-15-9-6-10-16-30/h5-23,34H,4,24H2,1-3H3,(H,40,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin II receptor, type 1 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283231
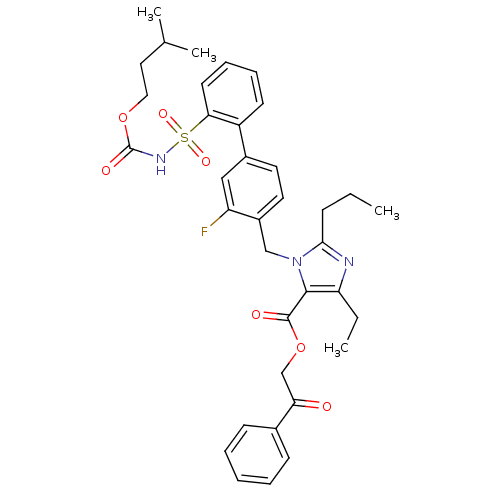
(Biphenylsulfonylcarbamate compound | CHEMBL70935)Show SMILES CCCc1nc(CC)c(C(=O)OCC(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C36H40FN3O7S/c1-5-12-33-38-30(6-2)34(35(42)47-23-31(41)25-13-8-7-9-14-25)40(33)22-27-18-17-26(21-29(27)37)28-15-10-11-16-32(28)48(44,45)39-36(43)46-20-19-24(3)4/h7-11,13-18,21,24H,5-6,12,19-20,22-23H2,1-4H3,(H,39,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283201
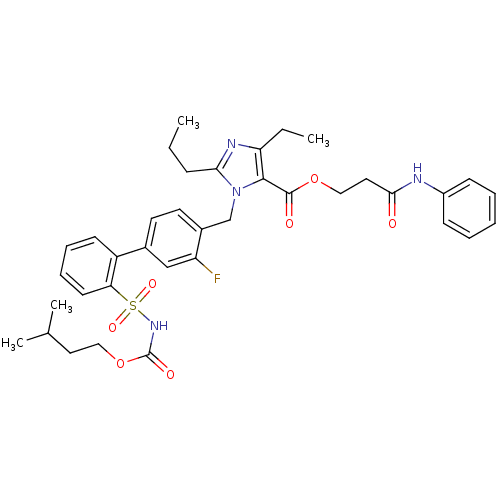
(Biphenylsulfonylcarbamate compound | CHEMBL305238)Show SMILES CCCc1nc(CC)c(C(=O)OCCC(=O)Nc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H43FN4O7S/c1-5-12-33-40-31(6-2)35(36(44)48-22-20-34(43)39-28-13-8-7-9-14-28)42(33)24-27-18-17-26(23-30(27)38)29-15-10-11-16-32(29)50(46,47)41-37(45)49-21-19-25(3)4/h7-11,13-18,23,25H,5-6,12,19-22,24H2,1-4H3,(H,39,43)(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283235

(Biphenylsulfonylcarbamate compound | CHEMBL305017)Show SMILES CCCc1nc(CC)c(C(=O)OCCN(C(=O)c2ccccc2)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C43H47FN4O7S/c1-5-15-39-45-37(6-2)40(42(50)54-27-25-47(34-18-11-8-12-19-34)41(49)31-16-9-7-10-17-31)48(39)29-33-23-22-32(28-36(33)44)35-20-13-14-21-38(35)56(52,53)46-43(51)55-26-24-30(3)4/h7-14,16-23,28,30H,5-6,15,24-27,29H2,1-4H3,(H,46,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283237
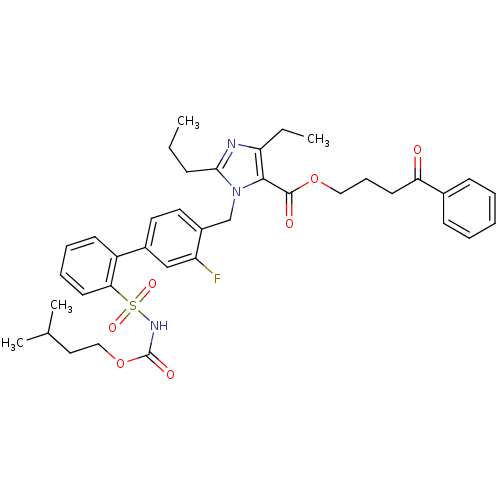
(Biphenylsulfonylcarbamate compound | CHEMBL70161)Show SMILES CCCc1nc(CC)c(C(=O)OCCCC(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H44FN3O7S/c1-5-13-35-40-32(6-2)36(37(44)48-22-12-17-33(43)27-14-8-7-9-15-27)42(35)25-29-20-19-28(24-31(29)39)30-16-10-11-18-34(30)50(46,47)41-38(45)49-23-21-26(3)4/h7-11,14-16,18-20,24,26H,5-6,12-13,17,21-23,25H2,1-4H3,(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283194

(Biphenylsulfonylcarbamate compound | CHEMBL69721)Show SMILES CCCc1nc(CC)c(C(=O)OCCCOc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H44FN3O7S/c1-5-13-34-39-32(6-2)35(36(42)47-22-12-21-46-29-14-8-7-9-15-29)41(34)25-28-19-18-27(24-31(28)38)30-16-10-11-17-33(30)49(44,45)40-37(43)48-23-20-26(3)4/h7-11,14-19,24,26H,5-6,12-13,20-23,25H2,1-4H3,(H,40,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283245
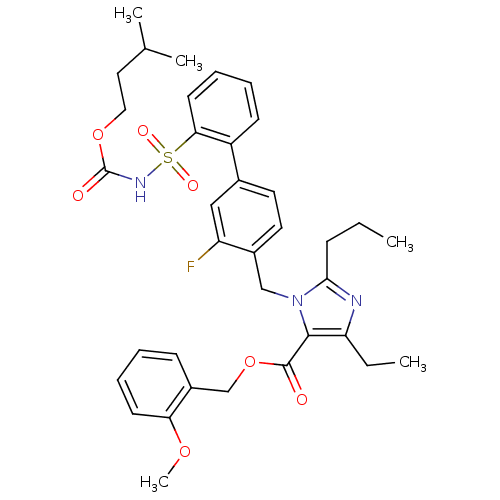
(Biphenylsulfonylcarbamate compound | CHEMBL70789)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2OC)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C36H42FN3O7S/c1-6-12-33-38-30(7-2)34(35(41)47-23-27-13-8-10-15-31(27)45-5)40(33)22-26-18-17-25(21-29(26)37)28-14-9-11-16-32(28)48(43,44)39-36(42)46-20-19-24(3)4/h8-11,13-18,21,24H,6-7,12,19-20,22-23H2,1-5H3,(H,39,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283219

(Biphenylsulfonylcarbamate compound | CHEMBL70843)Show SMILES CCCc1nc(CC)c(C(=O)OCCCN2C(=O)c3ccccc3C2=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C39H43FN4O8S/c1-5-12-34-41-32(6-2)35(38(47)51-21-11-20-43-36(45)29-14-7-8-15-30(29)37(43)46)44(34)24-27-18-17-26(23-31(27)40)28-13-9-10-16-33(28)53(49,50)42-39(48)52-22-19-25(3)4/h7-10,13-18,23,25H,5-6,11-12,19-22,24H2,1-4H3,(H,42,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50032358
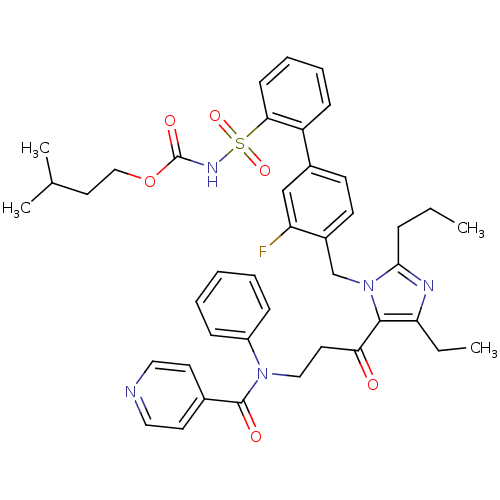
(CHEMBL430659 | isopentyloxy 2-[2-(4-{4-ethyl-5-[3-...)Show SMILES CCCc1nc(CC)c(C(=O)CCN(C(=O)c2ccncc2)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H46FN5O6S/c1-5-12-39-45-36(6-2)40(37(49)21-25-47(33-13-8-7-9-14-33)41(50)30-19-23-44-24-20-30)48(39)28-32-18-17-31(27-35(32)43)34-15-10-11-16-38(34)55(52,53)46-42(51)54-26-22-29(3)4/h7-11,13-20,23-24,27,29H,5-6,12,21-22,25-26,28H2,1-4H3,(H,46,51) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-[Sar1,Ile8]Ang II binding at rat angiotensin II (type 2) receptor. |
J Med Chem 38: 2938-45 (1995)
BindingDB Entry DOI: 10.7270/Q2639NRN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283223
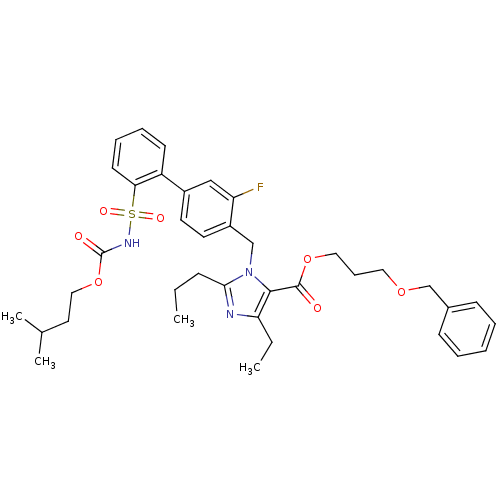
(Biphenylsulfonylcarbamate compound | CHEMBL70370)Show SMILES CCCc1nc(CC)c(C(=O)OCCCOCc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H46FN3O7S/c1-5-13-35-40-33(6-2)36(37(43)48-22-12-21-47-26-28-14-8-7-9-15-28)42(35)25-30-19-18-29(24-32(30)39)31-16-10-11-17-34(31)50(45,46)41-38(44)49-23-20-27(3)4/h7-11,14-19,24,27H,5-6,12-13,20-23,25-26H2,1-4H3,(H,41,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283213

(Biphenylsulfonylcarbamate compound | CHEMBL307318)Show SMILES CCCCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CC)c2C(=O)OCCC(=O)Nc2ccccc2)c(F)c1 Show InChI InChI=1S/C37H43FN4O7S/c1-4-7-13-22-49-37(45)41-50(46,47)32-18-12-11-17-29(32)26-19-20-27(30(38)24-26)25-42-33(14-5-2)40-31(6-3)35(42)36(44)48-23-21-34(43)39-28-15-9-8-10-16-28/h8-12,15-20,24H,4-7,13-14,21-23,25H2,1-3H3,(H,39,43)(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50041969
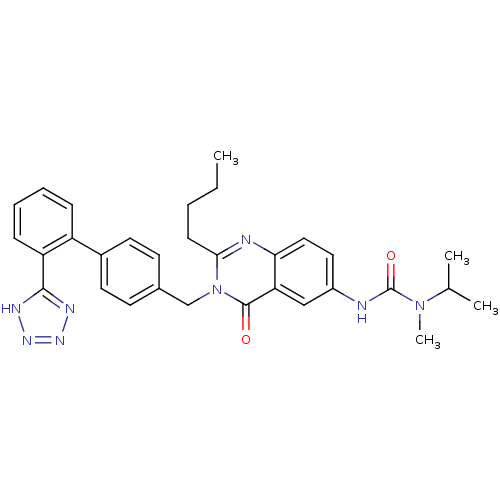
(3-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccc(NC(=O)N(C)C(C)C)cc2c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H34N8O2/c1-5-6-11-28-33-27-17-16-23(32-31(41)38(4)20(2)3)18-26(27)30(40)39(28)19-21-12-14-22(15-13-21)24-9-7-8-10-25(24)29-34-36-37-35-29/h7-10,12-18,20H,5-6,11,19H2,1-4H3,(H,32,41)(H,34,35,36,37) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against Angiotensin II receptor, type 1 in rabbit aorta |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030711

(CHEMBL338027 | L-163958 | Pentanoic acid {4-bromo-...)Show SMILES CCCCC(=O)Nc1ccc(Br)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O Show InChI InChI=1S/C33H37BrFN5O6S/c1-6-8-13-30(41)36-23-16-17-25(34)27(19-23)40-32(43)39(29(7-2)37-40)20-22-15-14-21(18-26(22)35)24-11-9-10-12-28(24)47(44,45)38-31(42)46-33(3,4)5/h9-12,14-19H,6-8,13,20H2,1-5H3,(H,36,41)(H,38,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin II receptor, type 2 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50032368
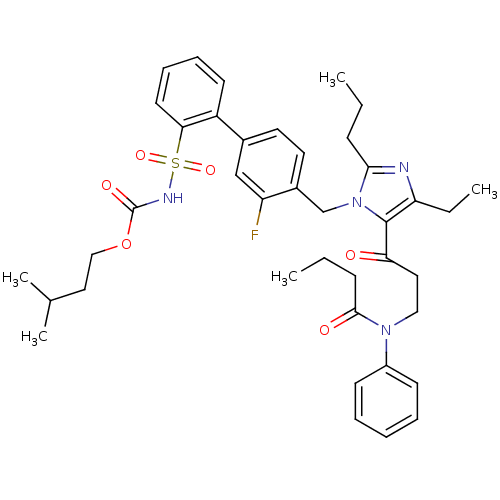
(CHEMBL98047 | butyloxy 2-[2-(4-{4-ethyl-5-[3-pheny...)Show SMILES CCCC(=O)N(CCC(=O)c1c(CC)nc(CCC)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C)c1ccccc1 Show InChI InChI=1S/C40H49FN4O6S/c1-6-14-37-42-34(8-3)39(35(46)22-24-44(38(47)15-7-2)31-16-10-9-11-17-31)45(37)27-30-21-20-29(26-33(30)41)32-18-12-13-19-36(32)52(49,50)43-40(48)51-25-23-28(4)5/h9-13,16-21,26,28H,6-8,14-15,22-25,27H2,1-5H3,(H,43,48) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-[Sar1,Ile8]Ang II binding at rat angiotensin II (type 2) receptor. |
J Med Chem 38: 2938-45 (1995)
BindingDB Entry DOI: 10.7270/Q2639NRN |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50032367

(CHEMBL317962 | isopentyloxy 2-[2-(4-{4-ethyl-5-[3-...)Show SMILES CCCc1nc(CC)c(C(=O)CCN(C(=O)c2cccnc2)c2ccccn2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C41H45FN6O6S/c1-5-12-38-45-34(6-2)39(35(49)19-23-47(37-16-9-10-22-44-37)40(50)30-13-11-21-43-26-30)48(38)27-31-18-17-29(25-33(31)42)32-14-7-8-15-36(32)55(52,53)46-41(51)54-24-20-28(3)4/h7-11,13-18,21-22,25-26,28H,5-6,12,19-20,23-24,27H2,1-4H3,(H,46,51) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-[Sar1,Ile8]Ang II binding at rat angiotensin II (type 2) receptor. |
J Med Chem 38: 2938-45 (1995)
BindingDB Entry DOI: 10.7270/Q2639NRN |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50032352
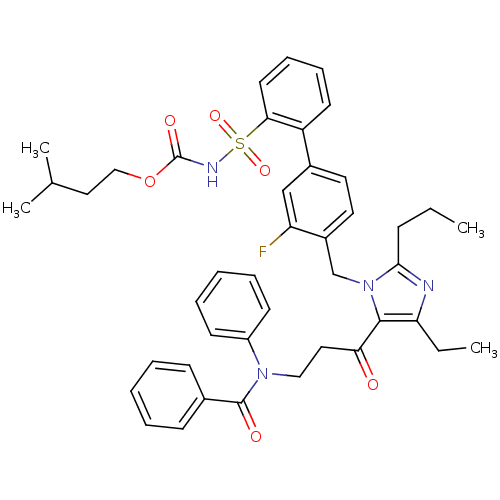
(CHEMBL98665 | butyloxy 2-[2-(4-{4-ethyl-5-[3-diphe...)Show SMILES CCCc1nc(CC)c(C(=O)CCN(C(=O)c2ccccc2)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C43H47FN4O6S/c1-5-15-40-45-37(6-2)41(38(49)24-26-47(34-18-11-8-12-19-34)42(50)31-16-9-7-10-17-31)48(40)29-33-23-22-32(28-36(33)44)35-20-13-14-21-39(35)55(52,53)46-43(51)54-27-25-30(3)4/h7-14,16-23,28,30H,5-6,15,24-27,29H2,1-4H3,(H,46,51) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-[Sar1,Ile8]Ang II binding at rat angiotensin II (type 2) receptor. |
J Med Chem 38: 2938-45 (1995)
BindingDB Entry DOI: 10.7270/Q2639NRN |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50032352

(CHEMBL98665 | butyloxy 2-[2-(4-{4-ethyl-5-[3-diphe...)Show SMILES CCCc1nc(CC)c(C(=O)CCN(C(=O)c2ccccc2)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C43H47FN4O6S/c1-5-15-40-45-37(6-2)41(38(49)24-26-47(34-18-11-8-12-19-34)42(50)31-16-9-7-10-17-31)48(40)29-33-23-22-32(28-36(33)44)35-20-13-14-21-39(35)55(52,53)46-43(51)54-27-25-30(3)4/h7-14,16-23,28,30H,5-6,15,24-27,29H2,1-4H3,(H,46,51) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-[Sar1,Ile8]Ang II binding at rat angiotensin II (type 2) receptor. |
J Med Chem 38: 2938-45 (1995)
BindingDB Entry DOI: 10.7270/Q2639NRN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283225

(Biphenylsulfonylcarbamate compound | CHEMBL302964)Show SMILES CCCc1nc(CC)c(C(=O)OCCCCOc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H46FN3O7S/c1-5-14-35-40-33(6-2)36(37(43)48-23-13-12-22-47-30-15-8-7-9-16-30)42(35)26-29-20-19-28(25-32(29)39)31-17-10-11-18-34(31)50(45,46)41-38(44)49-24-21-27(3)4/h7-11,15-20,25,27H,5-6,12-14,21-24,26H2,1-4H3,(H,41,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283209
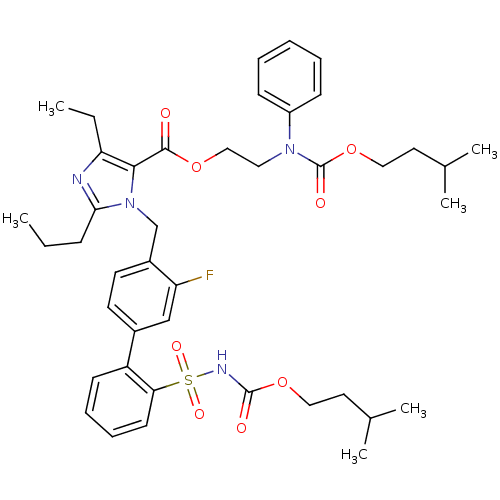
(Biphenylsulfonylcarbamate compound | CHEMBL303427)Show SMILES CCCc1nc(CC)c(C(=O)OCCN(C(=O)OCCC(C)C)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H53FN4O8S/c1-7-14-38-44-36(8-2)39(40(48)53-26-23-46(33-15-10-9-11-16-33)42(50)55-25-22-30(5)6)47(38)28-32-20-19-31(27-35(32)43)34-17-12-13-18-37(34)56(51,52)45-41(49)54-24-21-29(3)4/h9-13,15-20,27,29-30H,7-8,14,21-26,28H2,1-6H3,(H,45,49) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283200
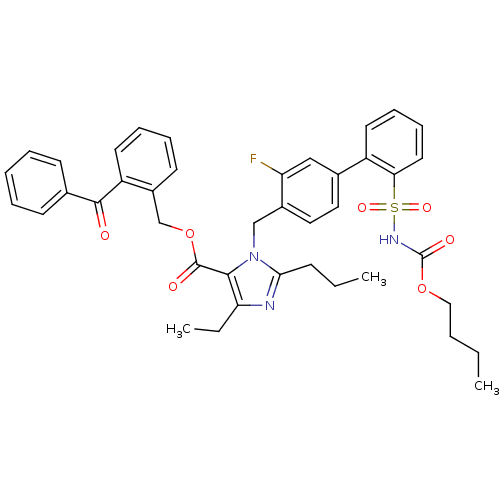
(Biphenylsulfonylcarbamate compound | CHEMBL71172)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CC)c2C(=O)OCc2ccccc2C(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C41H42FN3O7S/c1-4-7-24-51-41(48)44-53(49,50)36-21-14-13-19-32(36)29-22-23-30(34(42)25-29)26-45-37(15-5-2)43-35(6-3)38(45)40(47)52-27-31-18-11-12-20-33(31)39(46)28-16-9-8-10-17-28/h8-14,16-23,25H,4-7,15,24,26-27H2,1-3H3,(H,44,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50038189

(4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C30H28N4O3S/c1-4-27-32-28-20(2)18-21(3)31-29(28)34(27)19-22-14-16-23(17-15-22)25-12-8-9-13-26(25)38(36,37)33-30(35)24-10-6-5-7-11-24/h5-18H,4,19H2,1-3H3,(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against Angiotensin II receptor, type 1 in rabbit aorta |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283203

(Biphenylsulfonylcarbamate compound | CHEMBL70868)Show SMILES CCCCN(Cc1ccccc1)C(=O)CCCOC(=O)c1c(CC)nc(CCC)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C43H55FN4O7S/c1-6-9-25-47(29-32-17-11-10-12-18-32)40(49)21-15-26-54-42(50)41-37(8-3)45-39(16-7-2)48(41)30-34-23-22-33(28-36(34)44)35-19-13-14-20-38(35)56(52,53)46-43(51)55-27-24-31(4)5/h10-14,17-20,22-23,28,31H,6-9,15-16,21,24-27,29-30H2,1-5H3,(H,46,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283207
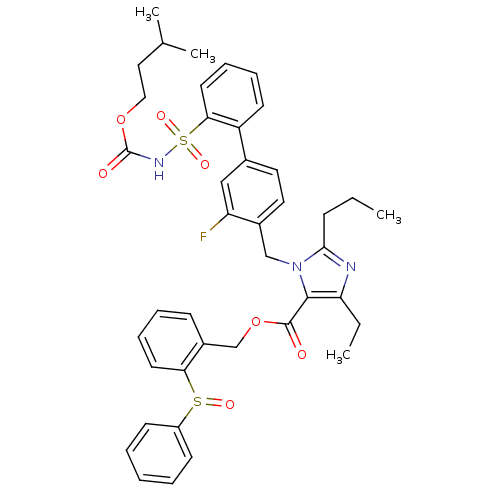
(Biphenylsulfonylcarbamate compound | CHEMBL73283)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2S(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C41H44FN3O7S2/c1-5-14-38-43-35(6-2)39(40(46)52-27-31-15-10-12-19-36(31)53(48)32-16-8-7-9-17-32)45(38)26-30-22-21-29(25-34(30)42)33-18-11-13-20-37(33)54(49,50)44-41(47)51-24-23-28(3)4/h7-13,15-22,25,28H,5-6,14,23-24,26-27H2,1-4H3,(H,44,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030711

(CHEMBL338027 | L-163958 | Pentanoic acid {4-bromo-...)Show SMILES CCCCC(=O)Nc1ccc(Br)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O Show InChI InChI=1S/C33H37BrFN5O6S/c1-6-8-13-30(41)36-23-16-17-25(34)27(19-23)40-32(43)39(29(7-2)37-40)20-22-15-14-21(18-26(22)35)24-11-9-10-12-28(24)47(44,45)38-31(42)46-33(3,4)5/h9-12,14-19H,6-8,13,20H2,1-5H3,(H,36,41)(H,38,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin II receptor, type 1 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283228
(Biphenylsulfonylcarbamate compound | CHEMBL430792)Show SMILES CCCc1nc(CC)c(C(=O)OCCCC(=O)Nc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H45FN4O7S/c1-5-13-34-41-32(6-2)36(37(45)49-22-12-18-35(44)40-29-14-8-7-9-15-29)43(34)25-28-20-19-27(24-31(28)39)30-16-10-11-17-33(30)51(47,48)42-38(46)50-23-21-26(3)4/h7-11,14-17,19-20,24,26H,5-6,12-13,18,21-23,25H2,1-4H3,(H,40,44)(H,42,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283215
(Biphenylsulfonylcarbamate compound | CHEMBL311253)Show SMILES CCCc1nc(CC)c(C(=O)OCCN2C(=O)c3ccccc3C2=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H41FN4O8S/c1-5-11-33-40-31(6-2)34(37(46)50-21-19-42-35(44)28-13-7-8-14-29(28)36(42)45)43(33)23-26-17-16-25(22-30(26)39)27-12-9-10-15-32(27)52(48,49)41-38(47)51-20-18-24(3)4/h7-10,12-17,22,24H,5-6,11,18-21,23H2,1-4H3,(H,41,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283214

(Biphenylsulfonylcarbamate compound | CHEMBL71639)Show SMILES CCCc1nc(CC)c(C(=O)OCCCSc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H44FN3O6S2/c1-5-13-34-39-32(6-2)35(36(42)46-21-12-23-48-29-14-8-7-9-15-29)41(34)25-28-19-18-27(24-31(28)38)30-16-10-11-17-33(30)49(44,45)40-37(43)47-22-20-26(3)4/h7-11,14-19,24,26H,5-6,12-13,20-23,25H2,1-4H3,(H,40,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283204

(Biphenylsulfonylcarbamate compound | CHEMBL311625)Show SMILES CCCc1nc(CC)c(C(=O)OCCCS(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H44FN3O7S2/c1-5-13-34-39-32(6-2)35(36(42)47-21-12-23-49(44)29-14-8-7-9-15-29)41(34)25-28-19-18-27(24-31(28)38)30-16-10-11-17-33(30)50(45,46)40-37(43)48-22-20-26(3)4/h7-11,14-19,24,26H,5-6,12-13,20-23,25H2,1-4H3,(H,40,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data