

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163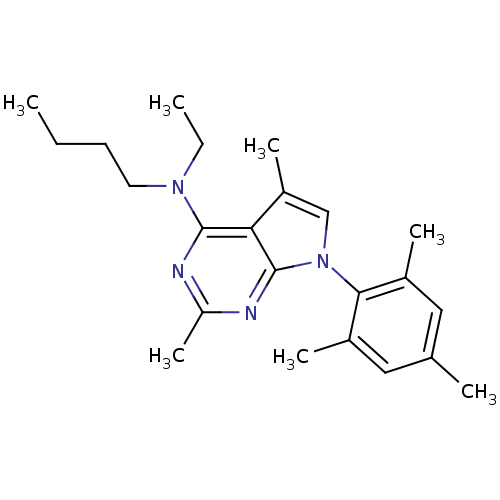 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135349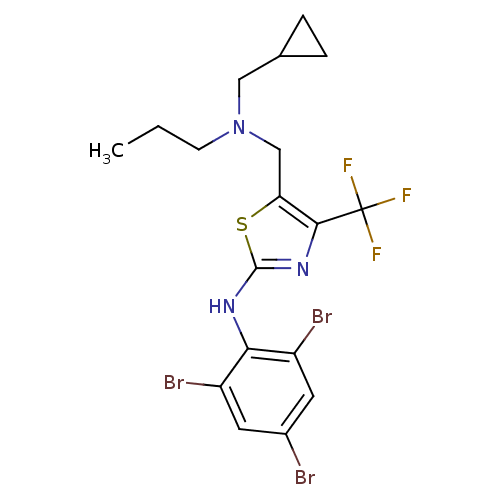 (CHEMBL341313 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135329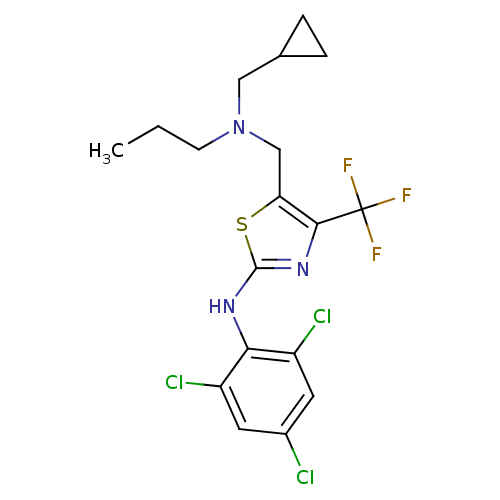 (CHEMBL128100 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135339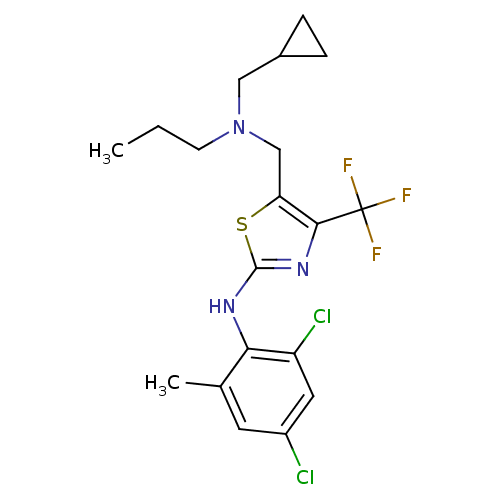 (CHEMBL128709 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50054245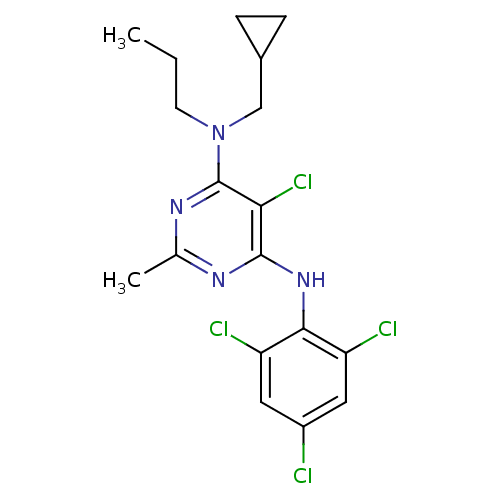 (5-Chloro-N-cyclopropylmethyl-2-methyl-N-propyl-N''...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135340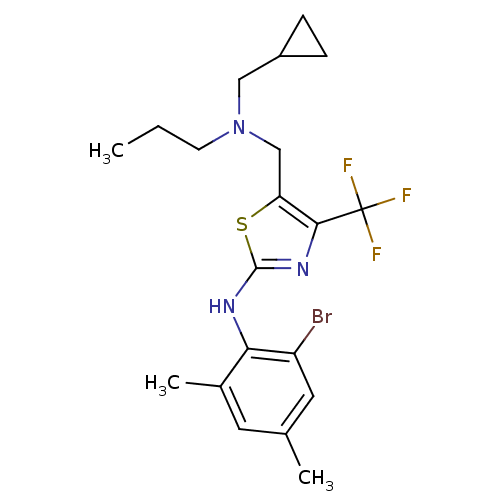 ((2-Bromo-4,6-dimethyl-phenyl)-{5-[(cyclopropylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135345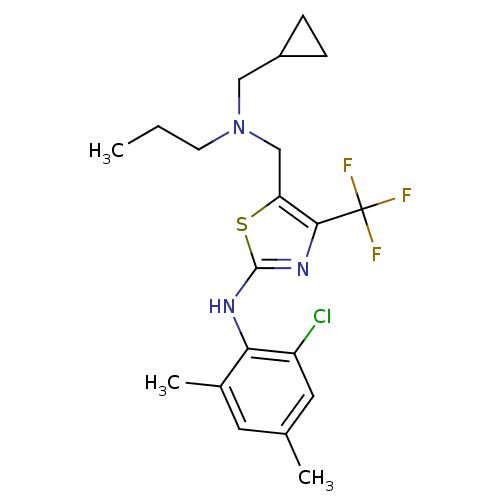 ((2-Chloro-4,6-dimethyl-phenyl)-{5-[(cyclopropylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135328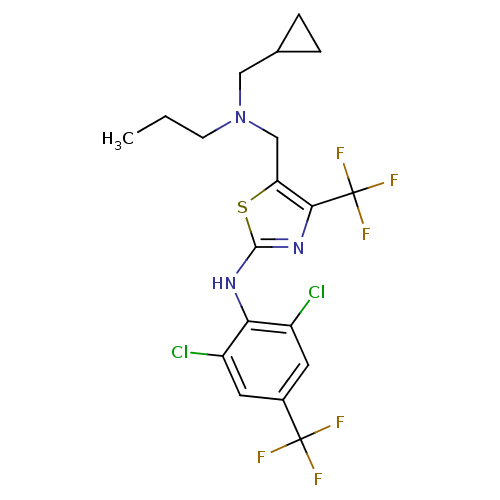 (CHEMBL131046 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135337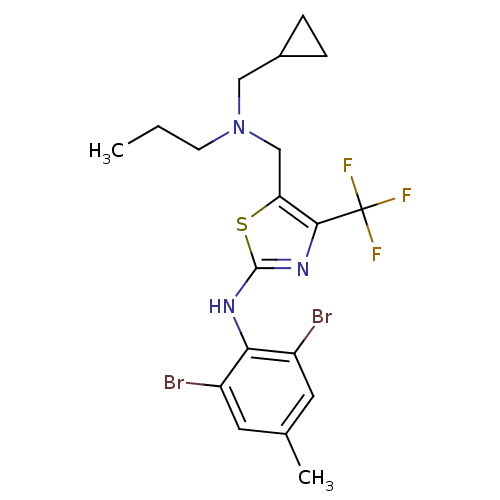 (CHEMBL131533 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135334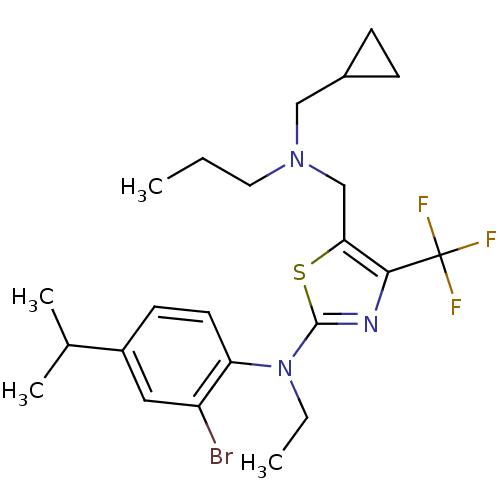 ((2-Bromo-4-isopropyl-phenyl)-{5-[(cyclopropylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135343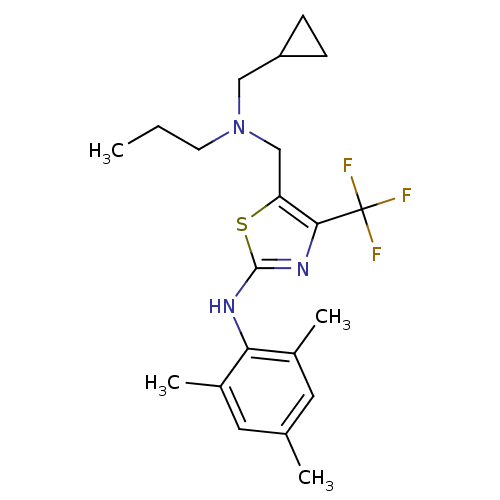 (CHEMBL128713 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135352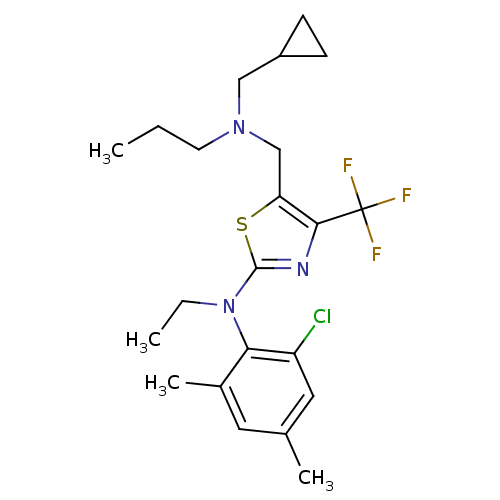 ((2-Chloro-4,6-dimethyl-phenyl)-{5-[(cyclopropylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135338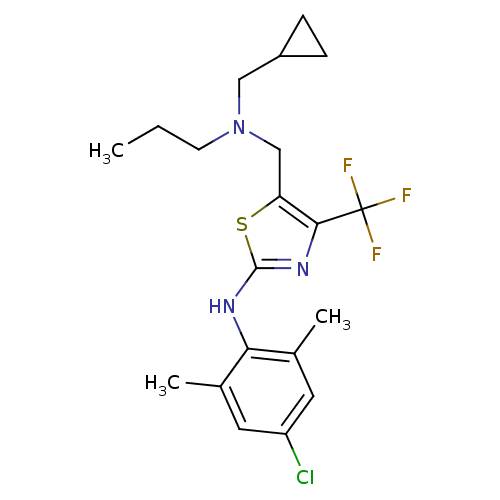 ((4-Chloro-2,6-dimethyl-phenyl)-{5-[(cyclopropylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135336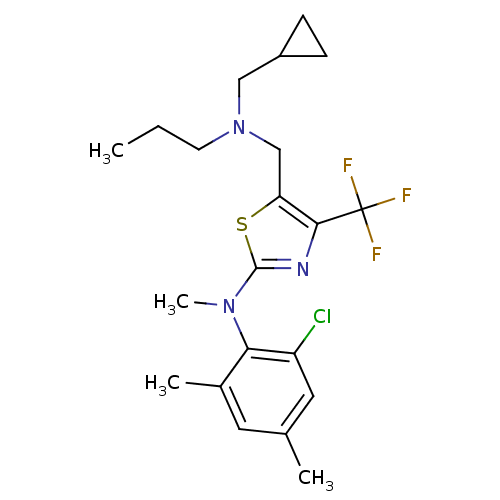 ((2-Chloro-4,6-dimethyl-phenyl)-{5-[(cyclopropylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135347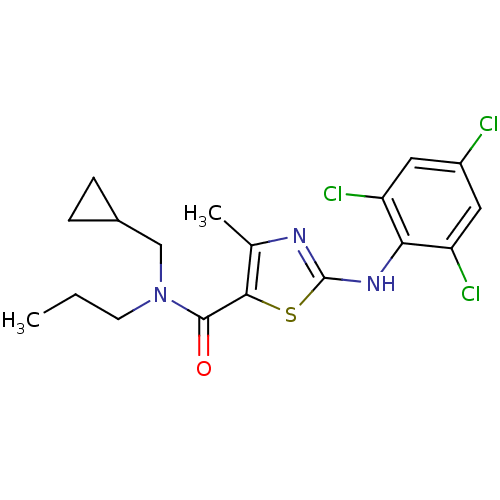 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135341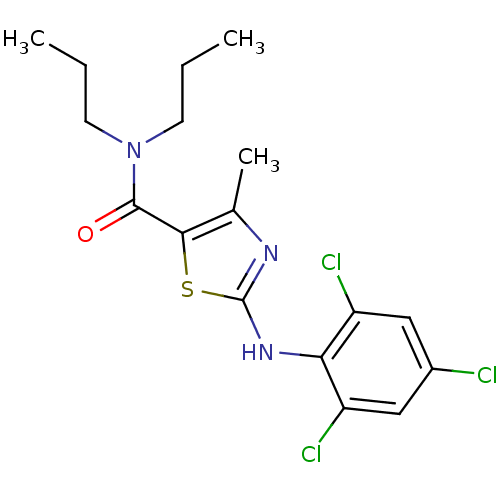 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135346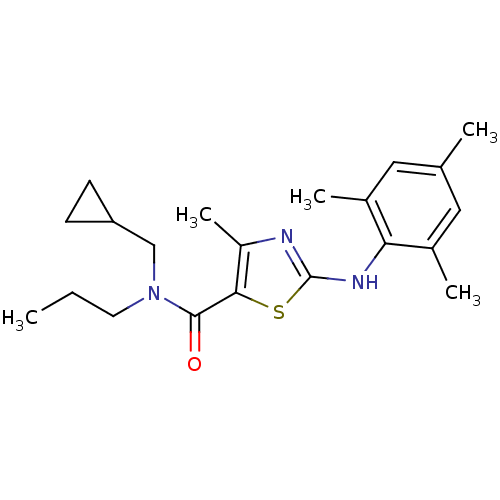 (4-Methyl-2-(2,4,6-trimethyl-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135344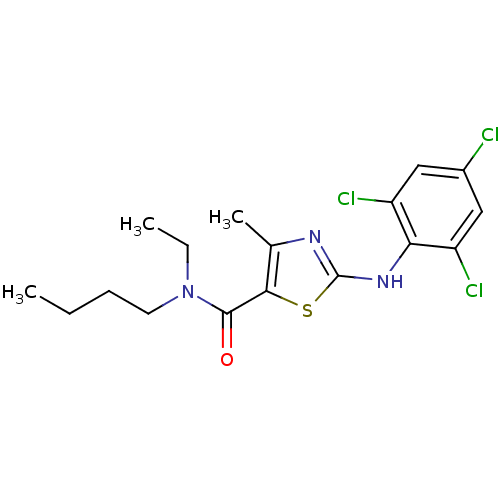 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135327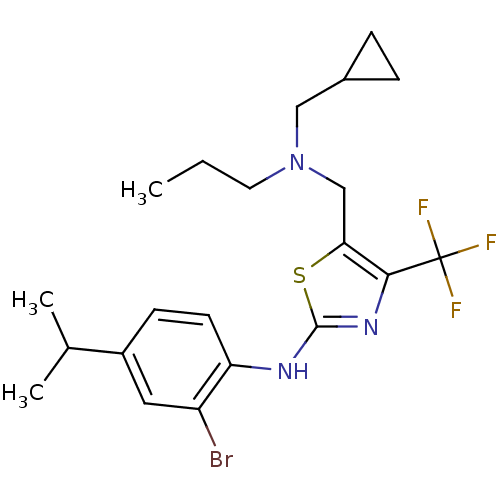 ((2-Bromo-4-isopropyl-phenyl)-{5-[(cyclopropylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135333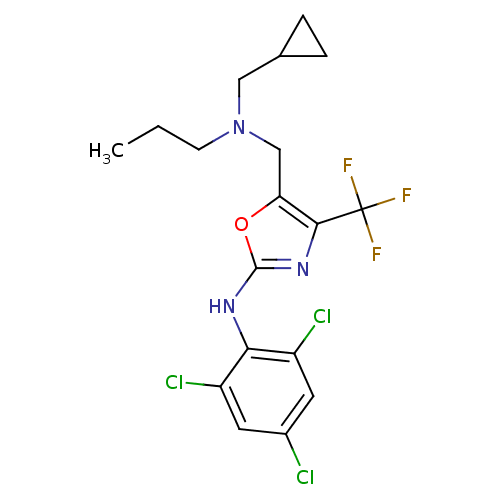 (CHEMBL128310 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135351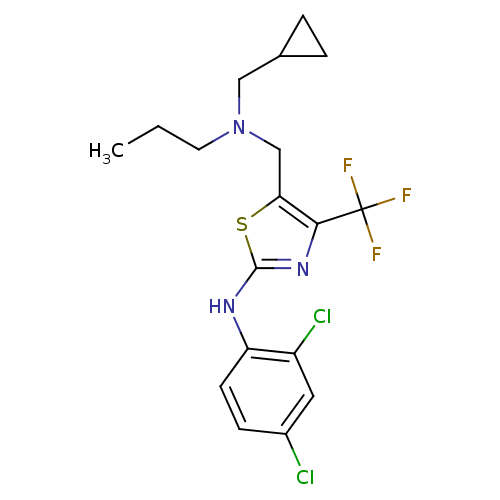 (CHEMBL338780 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135350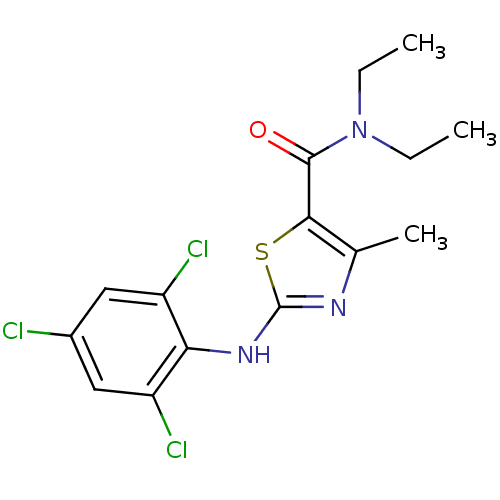 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135330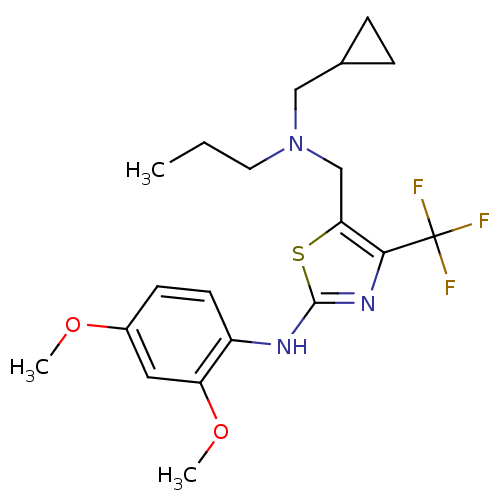 (CHEMBL127783 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135331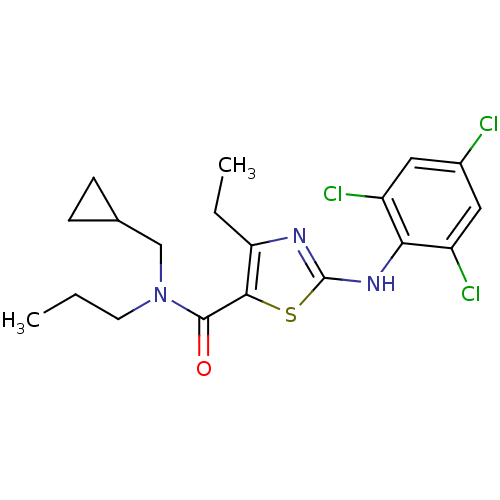 (4-Ethyl-2-(2,4,6-trichloro-phenylamino)-thiazole-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135348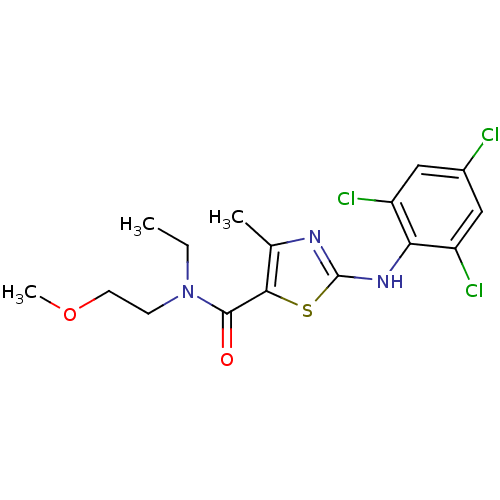 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135332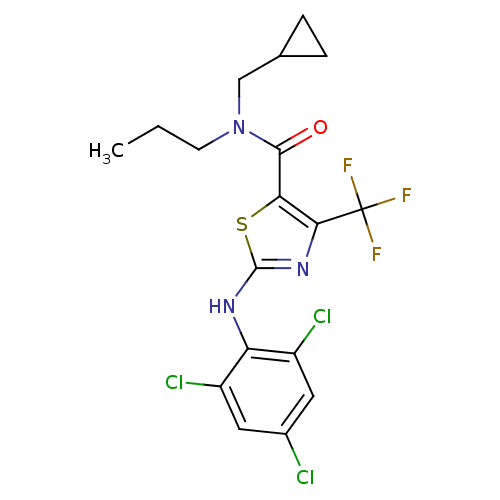 (2-(2,4,6-Trichloro-phenylamino)-4-trifluoromethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135342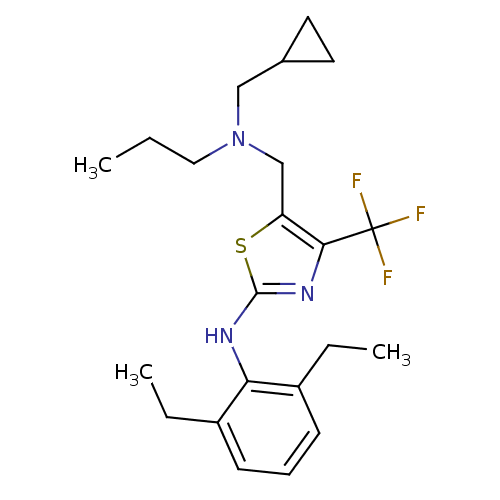 (CHEMBL340186 | {5-[(Cyclopropylmethyl-propyl-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50135335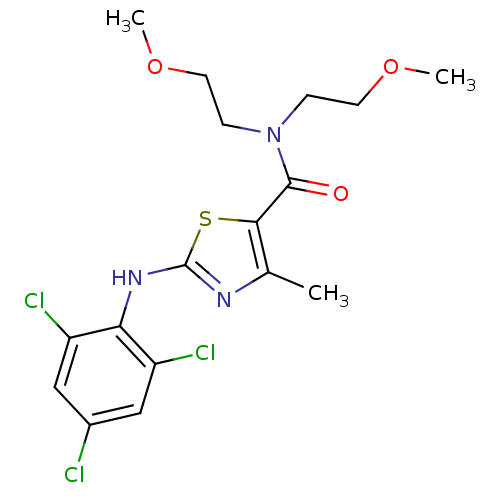 (4-Methyl-2-(2,4,6-trichloro-phenylamino)-thiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity by displacement of [125I]-Tyr-o-CRF from human corticotropin releasing factor receptor 1 expressed in IMR-32 human neuroblastoma cel... | Bioorg Med Chem Lett 13: 3997-4000 (2003) BindingDB Entry DOI: 10.7270/Q2MW2GJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461540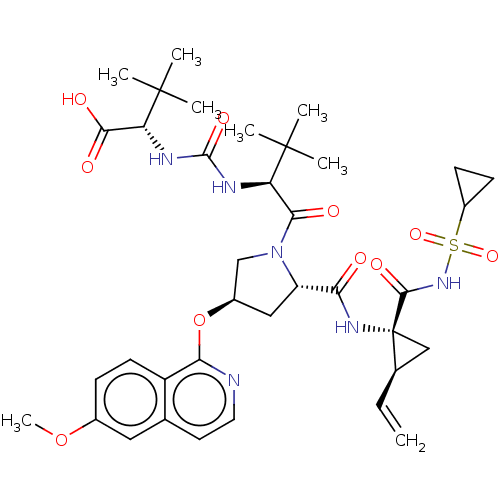 (CHEMBL4226033) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461545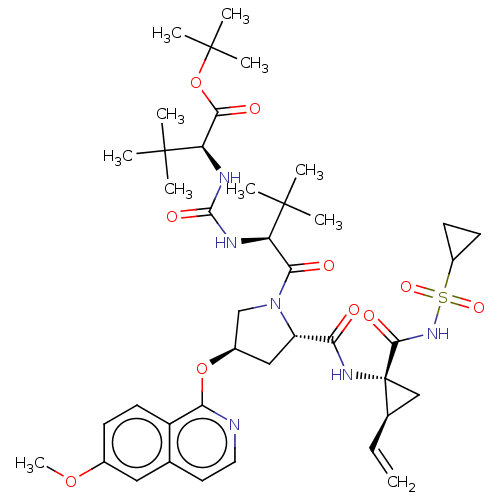 (CHEMBL4226876) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM592995 (US11578054, Example 1017) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | Citation and Details BindingDB Entry DOI: 10.7270/Q28P64FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461549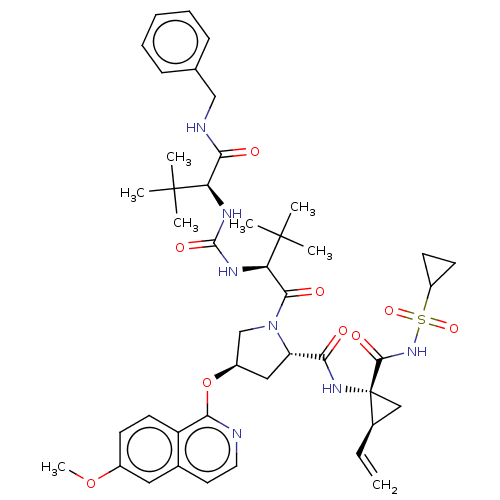 (CHEMBL4225009) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461552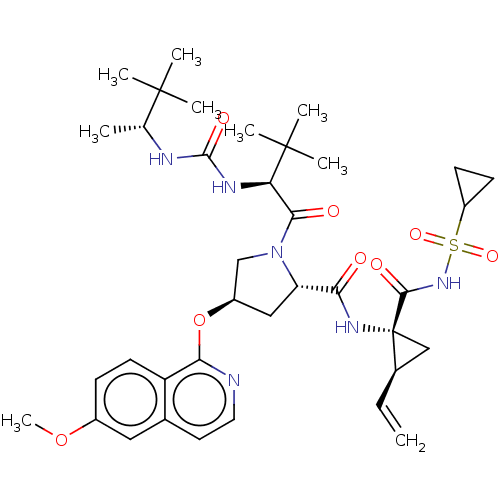 (CHEMBL4228791) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461537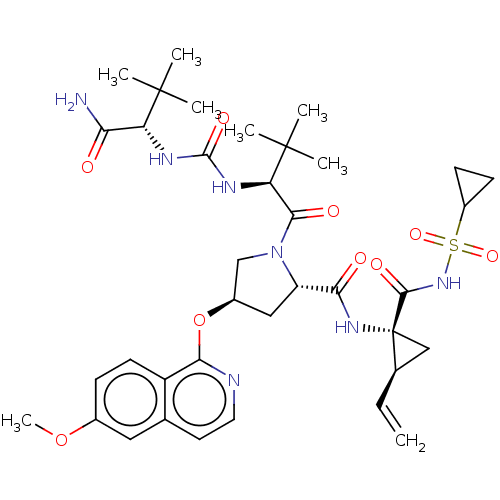 (CHEMBL4228440) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461556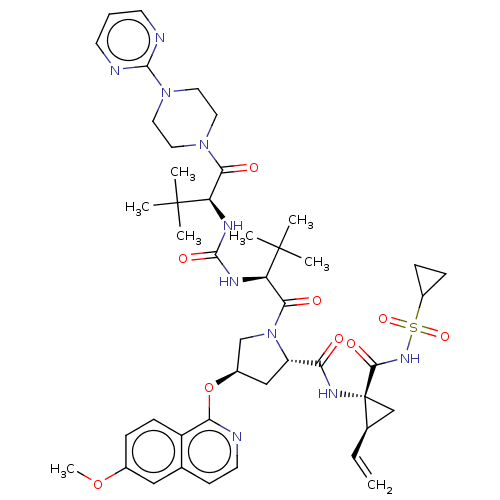 (CHEMBL4225149) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461547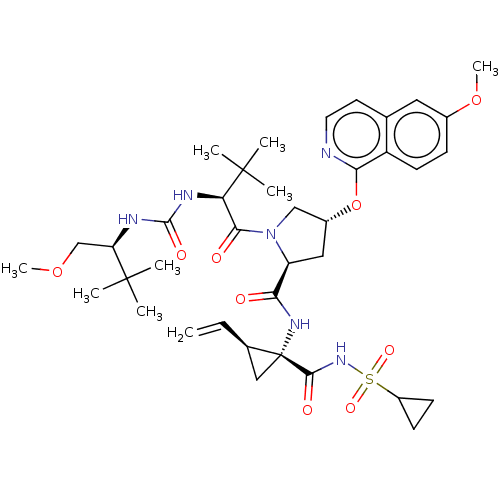 (CHEMBL4225051) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461555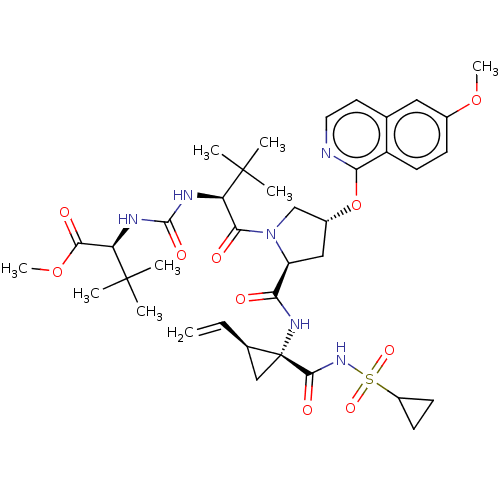 (CHEMBL4225768) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461554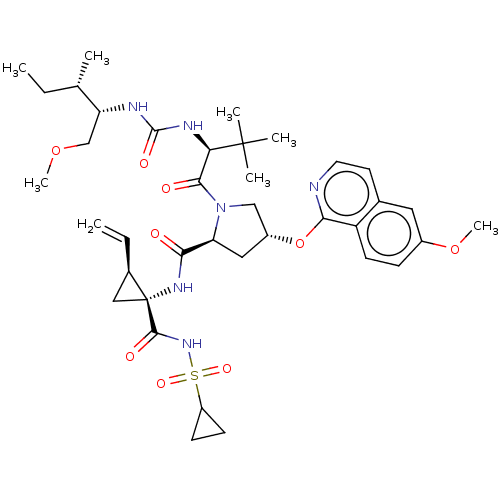 (CHEMBL4225514) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461539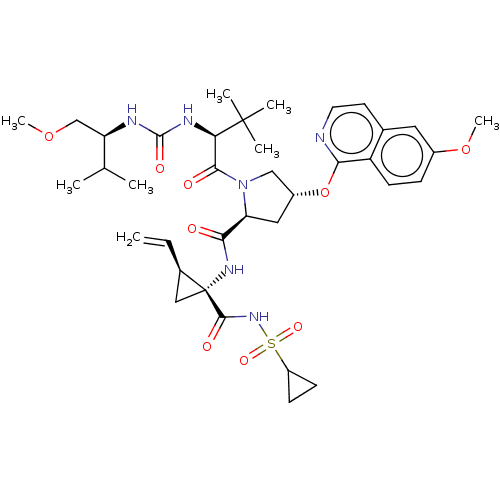 (CHEMBL4229110) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50461551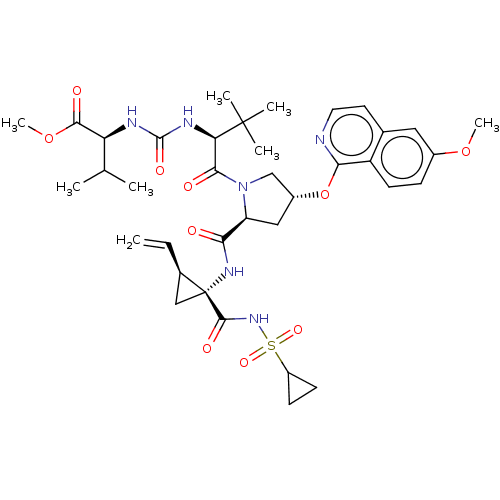 (CHEMBL4229052) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of N-terminal poly-His tagged recombinant HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) pLysS using HCV-FRET pep... | Bioorg Med Chem Lett 28: 1853-1859 (2018) Article DOI: 10.1016/j.bmcl.2018.04.009 BindingDB Entry DOI: 10.7270/Q2NP272Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM477037 (US10882844, Example 2015) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM477031 (US10882844, Example 2009) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM477027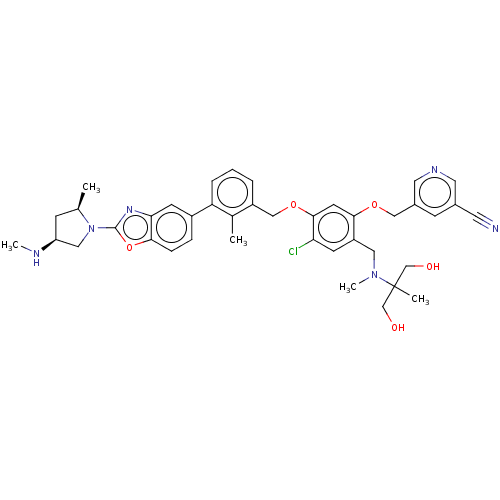 (US10882844, Example 2004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM477026 (US10882844, Example 2003) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM477014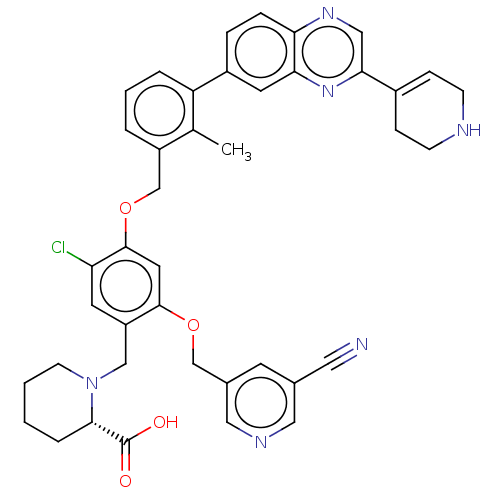 (US10882844, Example 1030) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM474566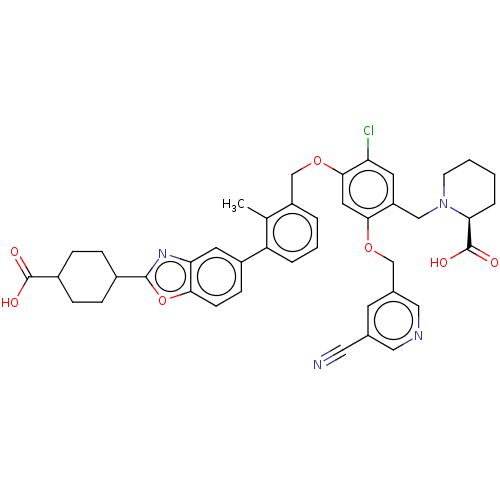 ((S)-1-(2-(4-carboxycyclohexyl)benzo[d]oxazol-5-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM474561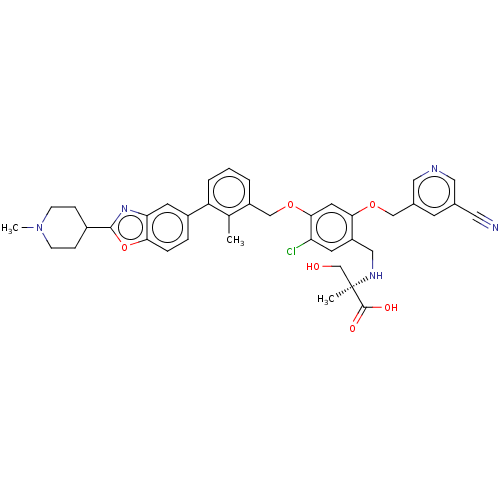 (US10882844, Example 1020) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM474557 (US10882844, Example 1017) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM474556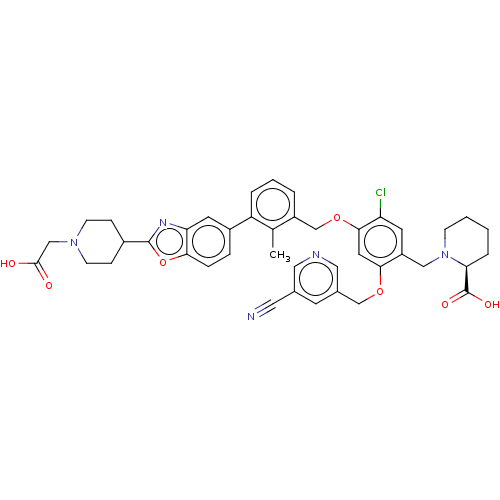 (US10882844, Example 1016) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM474547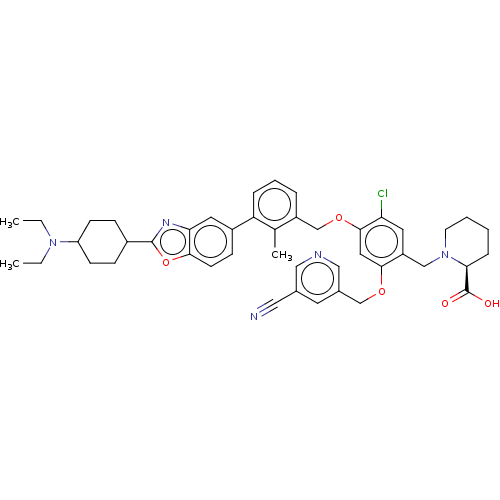 (US10882844, Example 1012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and... | US Patent US10882844 (2021) BindingDB Entry DOI: 10.7270/Q26113F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 912 total ) | Next | Last >> |