 Found 1476 hits with Last Name = 'ng' and Initial = 'py'
Found 1476 hits with Last Name = 'ng' and Initial = 'py' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507688
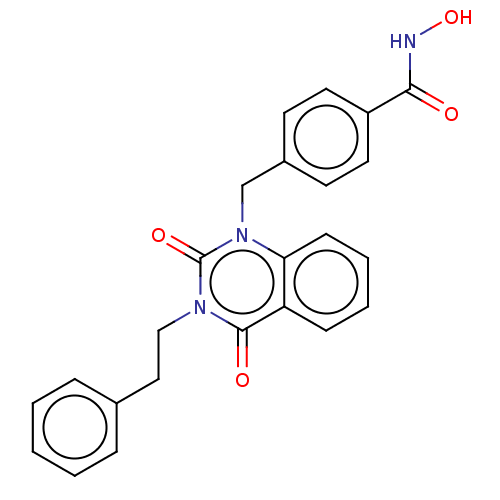
(CHEMBL4550214 | US11535598, Compound 5)Show SMILES ONC(=O)c1ccc(Cn2c3ccccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C24H21N3O4/c28-22(25-31)19-12-10-18(11-13-19)16-27-21-9-5-4-8-20(21)23(29)26(24(27)30)15-14-17-6-2-1-3-7-17/h1-13,31H,14-16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate ... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507695
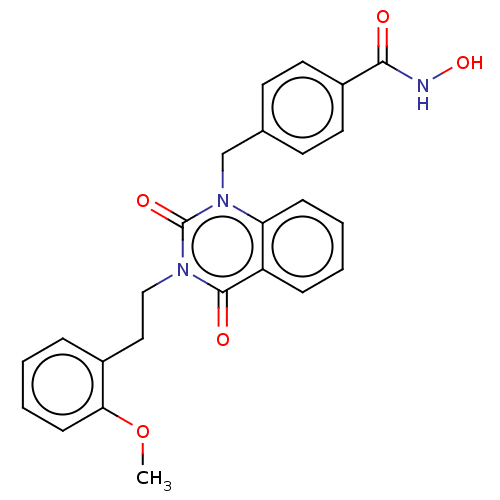
(CHEMBL4554270 | US11535598, Compound 15)Show SMILES COc1ccccc1CCn1c(=O)n(Cc2ccc(cc2)C(=O)NO)c2ccccc2c1=O Show InChI InChI=1S/C25H23N3O5/c1-33-22-9-5-2-6-18(22)14-15-27-24(30)20-7-3-4-8-21(20)28(25(27)31)16-17-10-12-19(13-11-17)23(29)26-32/h2-13,32H,14-16H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate preincubated... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507695

(CHEMBL4554270 | US11535598, Compound 15)Show SMILES COc1ccccc1CCn1c(=O)n(Cc2ccc(cc2)C(=O)NO)c2ccccc2c1=O Show InChI InChI=1S/C25H23N3O5/c1-33-22-9-5-2-6-18(22)14-15-27-24(30)20-7-3-4-8-21(20)28(25(27)31)16-17-10-12-19(13-11-17)23(29)26-32/h2-13,32H,14-16H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 CD2 expressed in Escherichia coli BL21 (RIL) using Boc-Lys (Ac)-AMC as substrate preincubated for 10 mins followed by subst... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate preincubated... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507688

(CHEMBL4550214 | US11535598, Compound 5)Show SMILES ONC(=O)c1ccc(Cn2c3ccccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C24H21N3O4/c28-22(25-31)19-12-10-18(11-13-19)16-27-21-9-5-4-8-20(21)23(29)26(24(27)30)15-14-17-6-2-1-3-7-17/h1-13,31H,14-16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human HDAC6 CD2 expressed in Escherichia coli BL21 (RIL) using Boc-Lys (Ac)-AMC as substrate preincubated for 10 mins follo... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507698
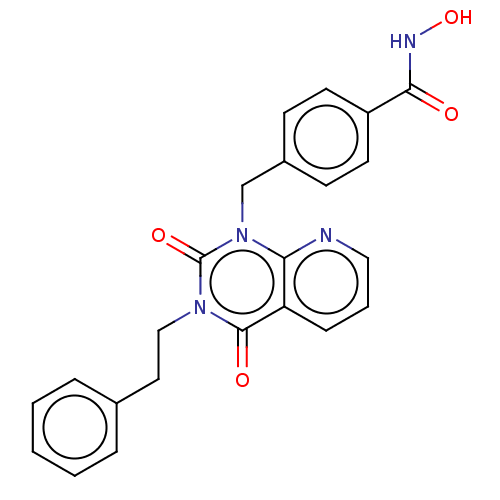
(CHEMBL4438057 | US11535598, Compound 12)Show SMILES ONC(=O)c1ccc(Cn2c3ncccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C23H20N4O4/c28-21(25-31)18-10-8-17(9-11-18)15-27-20-19(7-4-13-24-20)22(29)26(23(27)30)14-12-16-5-2-1-3-6-16/h1-11,13,31H,12,14-15H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate preincubated... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507698

(CHEMBL4438057 | US11535598, Compound 12)Show SMILES ONC(=O)c1ccc(Cn2c3ncccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C23H20N4O4/c28-21(25-31)18-10-8-17(9-11-18)15-27-20-19(7-4-13-24-20)22(29)26(23(27)30)14-12-16-5-2-1-3-6-16/h1-11,13,31H,12,14-15H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 CD2 expressed in Escherichia coli BL21 (RIL) using Boc-Lys (Ac)-AMC as substrate preincubated for 10 mins followed by subst... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM97563
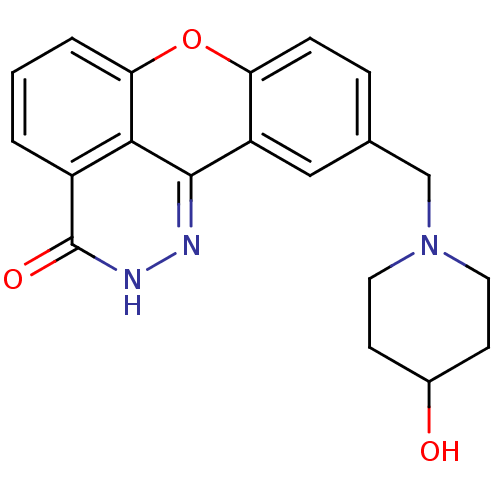
(US8470825, 4i)Show SMILES OC1CCN(Cc2ccc3oc4cccc5c4c(n[nH]c5=O)c3c2)CC1 Show InChI InChI=1S/C20H19N3O3/c24-13-6-8-23(9-7-13)11-12-4-5-16-15(10-12)19-18-14(20(25)22-21-19)2-1-3-17(18)26-16/h1-5,10,13,24H,6-9,11H2,(H,22,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50472300
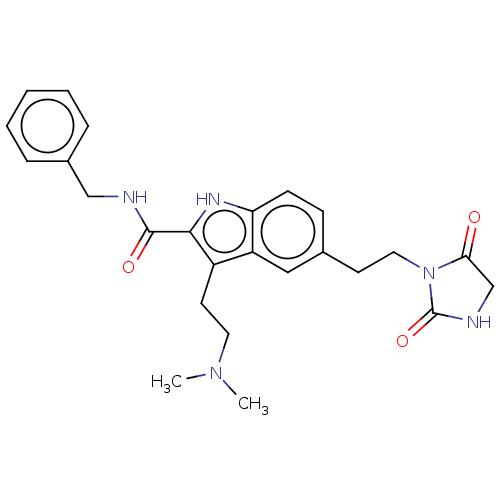
(CHEMBL84165)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)CNC3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H29N5O3/c1-29(2)12-11-19-20-14-17(10-13-30-22(31)16-27-25(30)33)8-9-21(20)28-23(19)24(32)26-15-18-6-4-3-5-7-18/h3-9,14,28H,10-13,15-16H2,1-2H3,(H,26,32)(H,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1D receptor in calf caudate homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50472300

(CHEMBL84165)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)CNC3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H29N5O3/c1-29(2)12-11-19-20-14-17(10-13-30-22(31)16-27-25(30)33)8-9-21(20)28-23(19)24(32)26-15-18-6-4-3-5-7-18/h3-9,14,28H,10-13,15-16H2,1-2H3,(H,26,32)(H,27,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor in rat cortex homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50472301
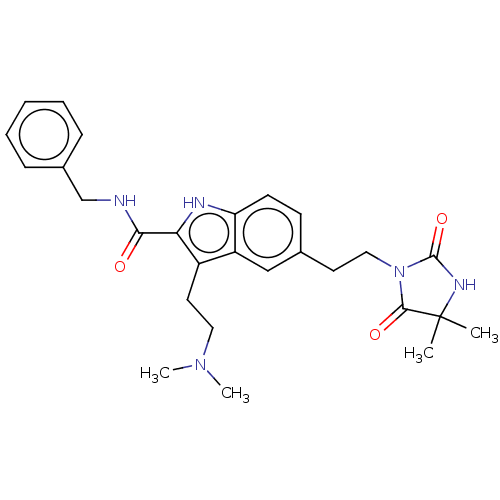
(CHEMBL86223)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)NC(C)(C)C3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C27H33N5O3/c1-27(2)25(34)32(26(35)30-27)15-12-18-10-11-22-21(16-18)20(13-14-31(3)4)23(29-22)24(33)28-17-19-8-6-5-7-9-19/h5-11,16,29H,12-15,17H2,1-4H3,(H,28,33)(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor in rat cortex homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50472301

(CHEMBL86223)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)NC(C)(C)C3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C27H33N5O3/c1-27(2)25(34)32(26(35)30-27)15-12-18-10-11-22-21(16-18)20(13-14-31(3)4)23(29-22)24(33)28-17-19-8-6-5-7-9-19/h5-11,16,29H,12-15,17H2,1-4H3,(H,28,33)(H,30,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1D receptor in calf caudate homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for 5-hydroxytryptamine 2C receptor was measured in rat cortex homogenates. |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Alpha-2 adrenergic receptor was measured in rat cortex homogenates. |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for 5-hydroxytryptamine 2A receptor was measured in rat cortex homogenates |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for 5-hydroxytryptamine 1A receptor was measured in rat cortex homogenates. |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for 5-hydroxytryptamine 1D receptor was measured in calf caudate homogenate |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Dopamine receptor D2 was measured in rat cortex homogenates |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Alpha-1 adrenergic receptor was measured in rat cortex homogenates |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50472301

(CHEMBL86223)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)NC(C)(C)C3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C27H33N5O3/c1-27(2)25(34)32(26(35)30-27)15-12-18-10-11-22-21(16-18)20(13-14-31(3)4)23(29-22)24(33)28-17-19-8-6-5-7-9-19/h5-11,16,29H,12-15,17H2,1-4H3,(H,28,33)(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1A receptor in rat cortex homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Dopamine receptor D1 was measured in rat cortex homogenates. |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50471285

(CHEMBL76480)Show SMILES CCOC(=O)c1[nH]c2ccc(CCN3C(=O)CNC3=O)cc2c1CCN(C)C Show InChI InChI=1S/C20H26N4O4/c1-4-28-19(26)18-14(8-9-23(2)3)15-11-13(5-6-16(15)22-18)7-10-24-17(25)12-21-20(24)27/h5-6,11,22H,4,7-10,12H2,1-3H3,(H,21,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Affinity pKi for Beta adrenergic receptor was measured in rat cortex homogenates |
J Med Chem 40: 2347-62 (1997)
Article DOI: 10.1021/jm9605849
BindingDB Entry DOI: 10.7270/Q2QZ2DN1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50472300

(CHEMBL84165)Show SMILES CN(C)CCc1c([nH]c2ccc(CCN3C(=O)CNC3=O)cc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H29N5O3/c1-29(2)12-11-19-20-14-17(10-13-30-22(31)16-27-25(30)33)8-9-21(20)28-23(19)24(32)26-15-18-6-4-3-5-7-18/h3-9,14,28H,10-13,15-16H2,1-2H3,(H,26,32)(H,27,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1A receptor in rat cortex homogenates. |
J Med Chem 42: 2504-26 (1999)
Article DOI: 10.1021/jm9706325
BindingDB Entry DOI: 10.7270/Q2VD7257 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM207624
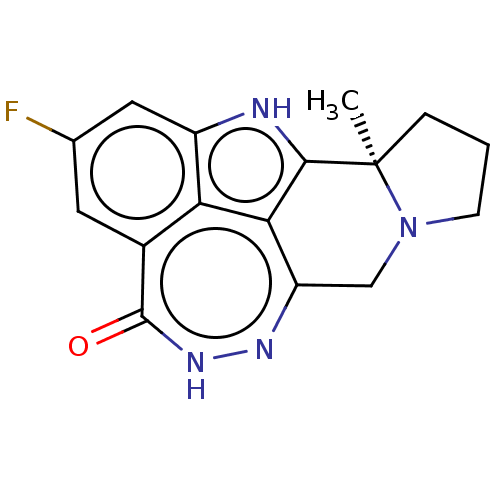
(US10501467, Example 69 | US9260440, 69 | US9617273...)Show SMILES C[C@]12CCCN1Cc1n[nH]c(=O)c3cc(F)cc4[nH]c2c1c34 |r| Show InChI InChI=1S/C16H13FN4O/c1-16-3-2-4-21(16)7-11-13-12-9(15(22)20-19-11)5-8(17)6-10(12)18-14(13)16/h5-6H,2-4,7H2,1H3/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50084621
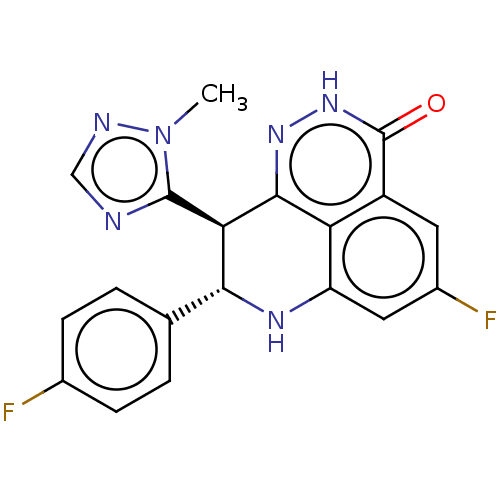
(BMN 673 | Talazoparib)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM207624

(US10501467, Example 69 | US9260440, 69 | US9617273...)Show SMILES C[C@]12CCCN1Cc1n[nH]c(=O)c3cc(F)cc4[nH]c2c1c34 |r| Show InChI InChI=1S/C16H13FN4O/c1-16-3-2-4-21(16)7-11-13-12-9(15(22)20-19-11)5-8(17)6-10(12)18-14(13)16/h5-6H,2-4,7H2,1H3/t16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM139658

(US8894989, 37)Show SMILES O=c1[nH]nc2[nH]c(CN3Cc4ccccc4C3)nc3cccc1c23 Show InChI InChI=1S/C18H15N5O/c24-18-13-6-3-7-14-16(13)17(21-22-18)20-15(19-14)10-23-8-11-4-1-2-5-12(11)9-23/h1-7H,8-10H2,(H,22,24)(H,19,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273535
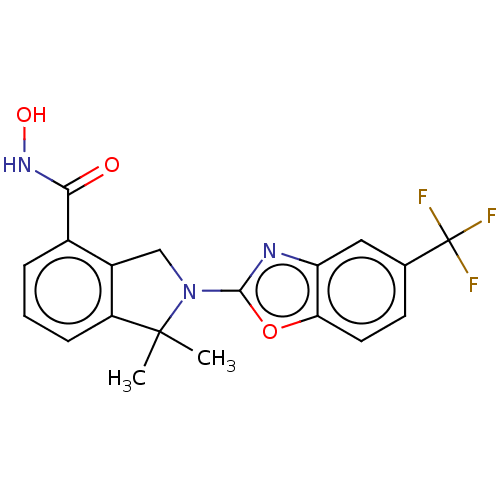
(CHEMBL4130288 | US11535607, Example 18-1)Show SMILES CC1(C)N(Cc2c1cccc2C(=O)NO)c1nc2cc(ccc2o1)C(F)(F)F Show InChI InChI=1S/C19H16F3N3O3/c1-18(2)13-5-3-4-11(16(26)24-27)12(13)9-25(18)17-23-14-8-10(19(20,21)22)6-7-15(14)28-17/h3-8,27H,9H2,1-2H3,(H,24,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Mus musculus (Mouse)) | BDBM139658

(US8894989, 37)Show SMILES O=c1[nH]nc2[nH]c(CN3Cc4ccccc4C3)nc3cccc1c23 Show InChI InChI=1S/C18H15N5O/c24-18-13-6-3-7-14-16(13)17(21-22-18)20-15(19-14)10-23-8-11-4-1-2-5-12(11)9-23/h1-7H,8-10H2,(H,22,24)(H,19,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50536939

(CHEMBL4569417)Show SMILES CC(C)(C)[Si](C)(C)OCCCN1CCN(CC1=O)C(=O)c1cc(Cn2c3ccccc3c(=O)[nH]c2=O)ccc1F Show InChI InChI=1S/C29H37FN4O5Si/c1-29(2,3)40(4,5)39-16-8-13-32-14-15-33(19-25(32)35)27(37)22-17-20(11-12-23(22)30)18-34-24-10-7-6-9-21(24)26(36)31-28(34)38/h6-7,9-12,17H,8,13-16,18-19H2,1-5H3,(H,31,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50536939

(CHEMBL4569417)Show SMILES CC(C)(C)[Si](C)(C)OCCCN1CCN(CC1=O)C(=O)c1cc(Cn2c3ccccc3c(=O)[nH]c2=O)ccc1F Show InChI InChI=1S/C29H37FN4O5Si/c1-29(2,3)40(4,5)39-16-8-13-32-14-15-33(19-25(32)35)27(37)22-17-20(11-12-23(22)30)18-34-24-10-7-6-9-21(24)26(36)31-28(34)38/h6-7,9-12,17H,8,13-16,18-19H2,1-5H3,(H,31,36,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50273537
(CHEMBL4128164 | US11535607, Example 50-5)Show InChI InChI=1S/C17H16N4O2/c22-16(20-23)12-5-6-13-10-21(8-7-11(13)9-12)17-18-14-3-1-2-4-15(14)19-17/h1-6,9,23H,7-8,10H2,(H,18,19)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
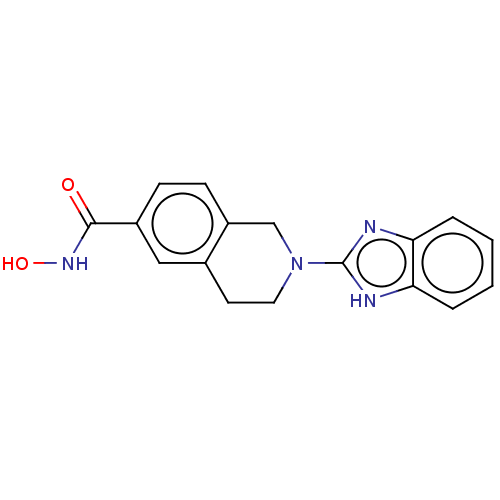
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273522
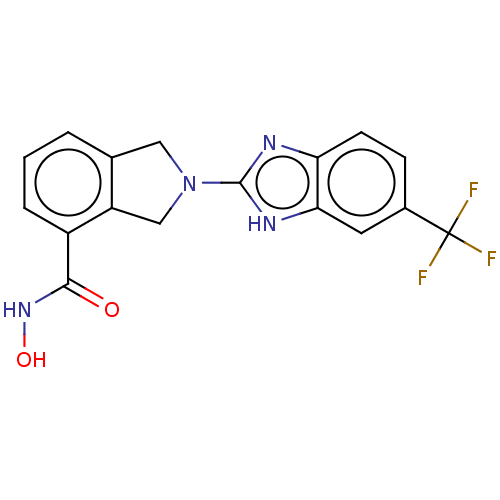
(CHEMBL4127743 | US11535607, Example 12-1)Show SMILES ONC(=O)c1cccc2CN(Cc12)c1nc2ccc(cc2[nH]1)C(F)(F)F Show InChI InChI=1S/C17H13F3N4O2/c18-17(19,20)10-4-5-13-14(6-10)22-16(21-13)24-7-9-2-1-3-11(12(9)8-24)15(25)23-26/h1-6,26H,7-8H2,(H,21,22)(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273525
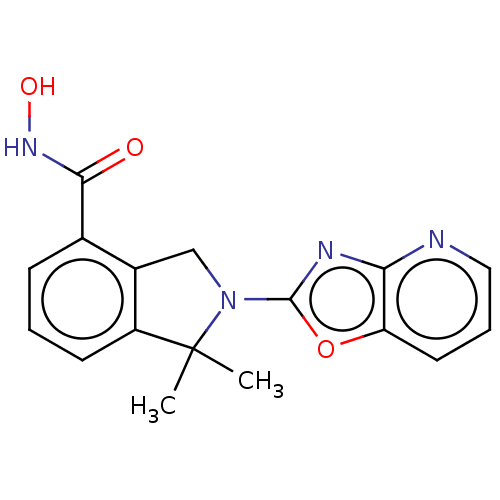
(CHEMBL4128972 | US11535607, Example 18-2)Show InChI InChI=1S/C17H16N4O3/c1-17(2)12-6-3-5-10(15(22)20-23)11(12)9-21(17)16-19-14-13(24-16)7-4-8-18-14/h3-8,23H,9H2,1-2H3,(H,20,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273553
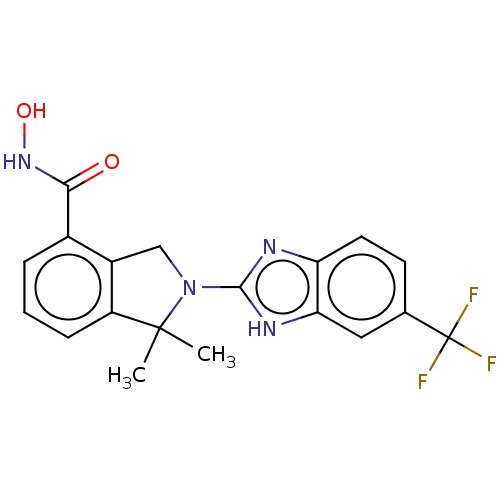
(CHEMBL4127020 | US10508088, ID HDTK028 | US1153560...)Show SMILES CC1(C)N(Cc2c1cccc2C(=O)NO)c1nc2ccc(cc2[nH]1)C(F)(F)F Show InChI InChI=1S/C19H17F3N4O2/c1-18(2)13-5-3-4-11(16(27)25-28)12(13)9-26(18)17-23-14-7-6-10(19(20,21)22)8-15(14)24-17/h3-8,28H,9H2,1-2H3,(H,23,24)(H,25,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273520
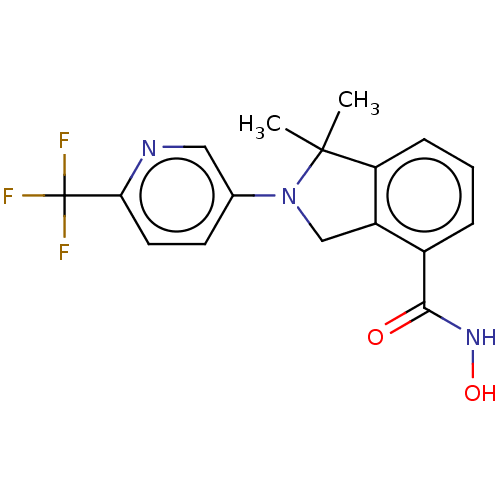
(CHEMBL4127735 | US11535607, Example 22-3)Show SMILES CC1(C)N(Cc2c1cccc2C(=O)NO)c1ccc(nc1)C(F)(F)F Show InChI InChI=1S/C17H16F3N3O2/c1-16(2)13-5-3-4-11(15(24)22-25)12(13)9-23(16)10-6-7-14(21-8-10)17(18,19)20/h3-8,25H,9H2,1-2H3,(H,22,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273553

(CHEMBL4127020 | US10508088, ID HDTK028 | US1153560...)Show SMILES CC1(C)N(Cc2c1cccc2C(=O)NO)c1nc2ccc(cc2[nH]1)C(F)(F)F Show InChI InChI=1S/C19H17F3N4O2/c1-18(2)13-5-3-4-11(16(27)25-28)12(13)9-26(18)17-23-14-7-6-10(19(20,21)22)8-15(14)24-17/h3-8,28H,9H2,1-2H3,(H,23,24)(H,25,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.
US Patent
| Assay Description
This example describes in vitro inhibition properties of exemplary HDAC11 inhibitors for various HDACs. HDAC inhibition assays were performed using a... |
US Patent US10508088 (2019)
BindingDB Entry DOI: 10.7270/Q2280B04 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM421500

(US10508088, Example 1-1 | US10508088, ID HDTK010 |...)Show InChI InChI=1S/C14H9Cl2N3O3/c15-9-5-11-12(6-10(9)16)22-14(18-11)17-8-3-1-2-7(4-8)13(20)19-21/h1-6,21H,(H,17,18)(H,19,20) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.
US Patent
| Assay Description
This example describes in vitro inhibition properties of exemplary HDAC11 inhibitors for various HDACs. HDAC inhibition assays were performed using a... |
US Patent US10508088 (2019)
BindingDB Entry DOI: 10.7270/Q2280B04 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273526
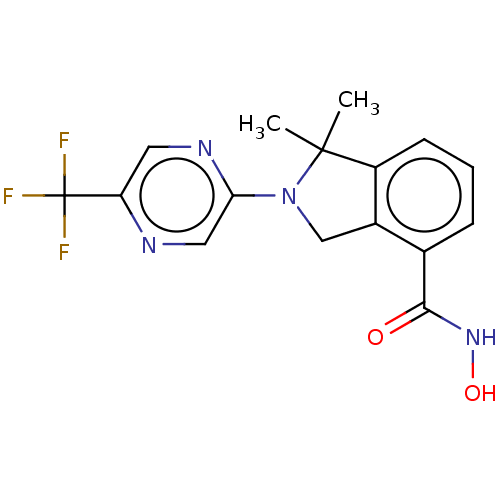
(CHEMBL4126661 | US11535607, Example 22-8)Show SMILES CC1(C)N(Cc2c1cccc2C(=O)NO)c1cnc(cn1)C(F)(F)F Show InChI InChI=1S/C16H15F3N4O2/c1-15(2)11-5-3-4-9(14(24)22-25)10(11)8-23(15)13-7-20-12(6-21-13)16(17,18)19/h3-7,25H,8H2,1-2H3,(H,22,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507688

(CHEMBL4550214 | US11535598, Compound 5)Show SMILES ONC(=O)c1ccc(Cn2c3ccccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C24H21N3O4/c28-22(25-31)19-12-10-18(11-13-19)16-27-21-9-5-4-8-20(21)23(29)26(24(27)30)15-14-17-6-2-1-3-7-17/h1-13,31H,14-16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using fluorogenic peptide RHKKAc as substrate af... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50273524
(CHEMBL4129402 | US11535607, Example 50-4)Show InChI InChI=1S/C17H16N4O2/c22-16(20-23)12-6-5-11-7-8-21(10-13(11)9-12)17-18-14-3-1-2-4-15(14)19-17/h1-6,9,23H,7-8,10H2,(H,18,19)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50288530
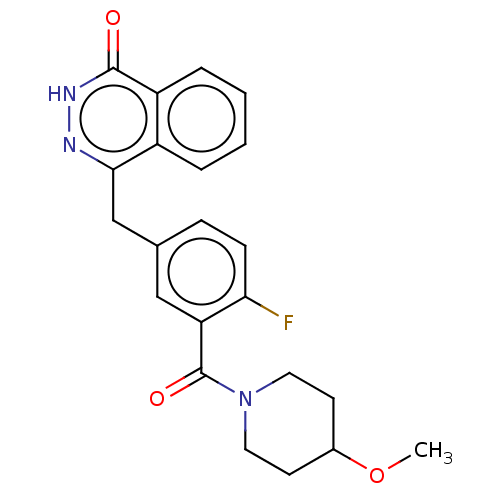
(CHEMBL4098253)Show SMILES COC1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C22H22FN3O3/c1-29-15-8-10-26(11-9-15)22(28)18-12-14(6-7-19(18)23)13-20-16-4-2-3-5-17(16)21(27)25-24-20/h2-7,12,15H,8-11,13H2,1H3,(H,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507696

(CHEMBL4462263)Show SMILES ONC(=O)c1ccc(Cn2c3ccccc3c(=O)n(CCc3ccc(O)cc3)c2=O)cc1 Show InChI InChI=1S/C24H21N3O5/c28-19-11-7-16(8-12-19)13-14-26-23(30)20-3-1-2-4-21(20)27(24(26)31)15-17-5-9-18(10-6-17)22(29)25-32/h1-12,28,32H,13-15H2,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using fluorogenic peptide RHKKAc as substrate af... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273538
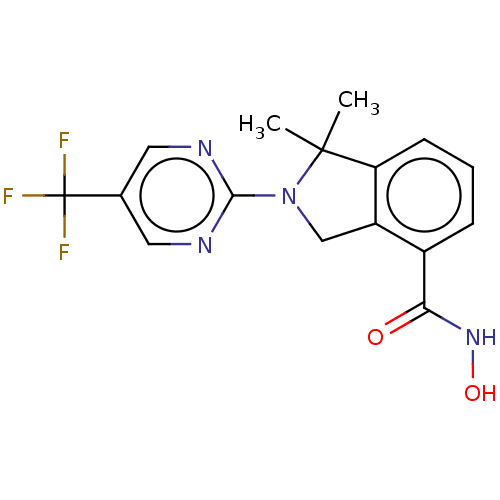
(CHEMBL4127894 | US11535607, Example 22-6)Show SMILES CC1(C)N(Cc2c1cccc2C(=O)NO)c1ncc(cn1)C(F)(F)F Show InChI InChI=1S/C16H15F3N4O2/c1-15(2)12-5-3-4-10(13(24)22-25)11(12)8-23(15)14-20-6-9(7-21-14)16(17,18)19/h3-7,25H,8H2,1-2H3,(H,22,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273554
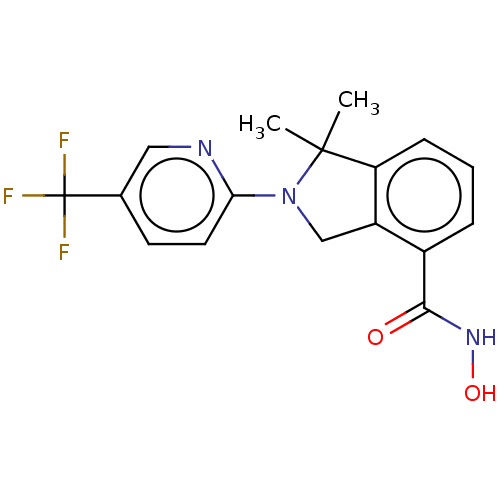
(CHEMBL4129134 | US11535607, Example 22-2)Show SMILES CC1(C)N(Cc2c1cccc2C(=O)NO)c1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C17H16F3N3O2/c1-16(2)13-5-3-4-11(15(24)22-25)12(13)9-23(16)14-7-6-10(8-21-14)17(18,19)20/h3-8,25H,9H2,1-2H3,(H,22,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Mus musculus) | BDBM50445795

(CHEMBL3104818)Show SMILES Cc1nc(sc1C(=O)NCc1cccnc1)N1CCN(Cc2ccc(F)cc2)C1=O Show InChI InChI=1S/C21H20FN5O2S/c1-14-18(19(28)24-12-16-3-2-8-23-11-16)30-20(25-14)27-10-9-26(21(27)29)13-15-4-6-17(22)7-5-15/h2-8,11H,9-10,12-13H2,1H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SCD1 in mouse liver microsomes |
Bioorg Med Chem Lett 24: 520-5 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.036
BindingDB Entry DOI: 10.7270/Q2QJ7JRS |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50613638
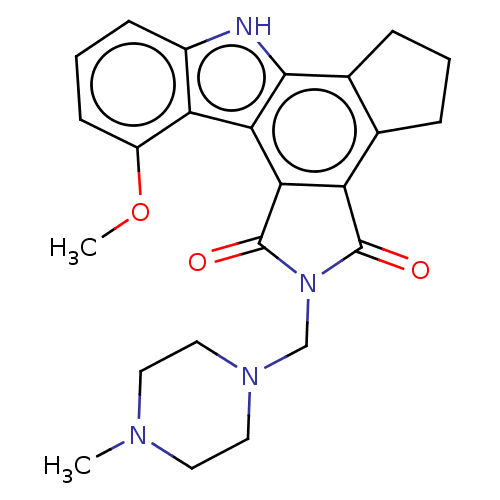
(CEP-9722 | Cep 9722 | Cep-9722)Show SMILES COc1cccc2[nH]c3c4CCCc4c4C(=O)N(CN5CCN(C)CC5)C(=O)c4c3c12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273549
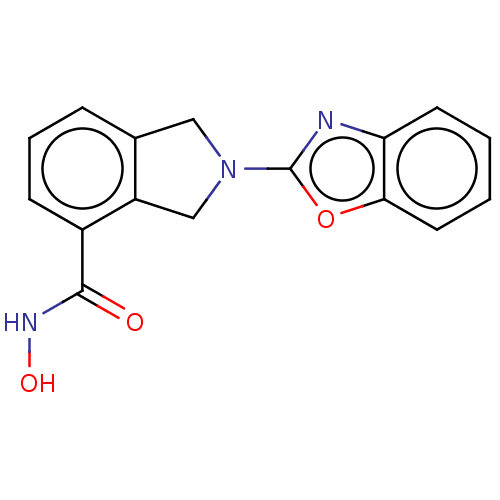
(CHEMBL4129266 | US11535607, Example 15-1)Show InChI InChI=1S/C16H13N3O3/c20-15(18-21)11-5-3-4-10-8-19(9-12(10)11)16-17-13-6-1-2-7-14(13)22-16/h1-7,21H,8-9H2,(H,18,20) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273551
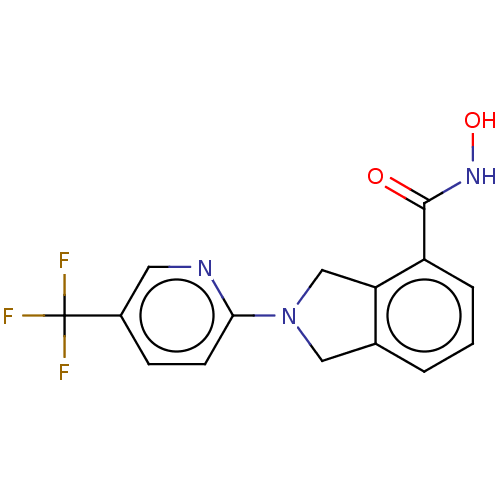
(CHEMBL4129143 | US11535607, Example 45-1)Show InChI InChI=1S/C15H12F3N3O2/c16-15(17,18)10-4-5-13(19-6-10)21-7-9-2-1-3-11(12(9)8-21)14(22)20-23/h1-6,23H,7-8H2,(H,20,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data