

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50442257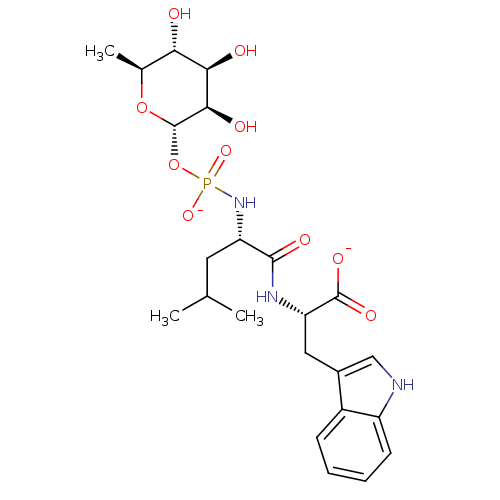 (CHEMBL2028192) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin using FA-Fly-Leu-NH2 as substrate at pH 7.5 after 15 mins by Henderson plot analysis | Bioorg Med Chem 21: 6778-87 (2013) Article DOI: 10.1016/j.bmc.2013.07.052 BindingDB Entry DOI: 10.7270/Q2CF9RJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50442257 (CHEMBL2028192) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin | Bioorg Med Chem 21: 6778-87 (2013) Article DOI: 10.1016/j.bmc.2013.07.052 BindingDB Entry DOI: 10.7270/Q2CF9RJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50442256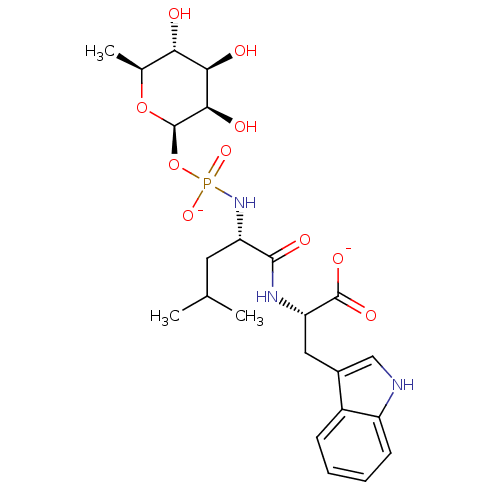 (CHEMBL2442111) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin using FA-Fly-Leu-NH2 as substrate at pH 7.5 after 15 mins by Henderson plot analysis | Bioorg Med Chem 21: 6778-87 (2013) Article DOI: 10.1016/j.bmc.2013.07.052 BindingDB Entry DOI: 10.7270/Q2CF9RJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50178352 (CHEMBL3814457) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of His-tagged full length human recombinant SPHK1 expressed in baculovirus infected fall armyworm Sf9 cell expression system using sphingo... | Bioorg Med Chem 24: 3218-30 (2016) Article DOI: 10.1016/j.bmc.2016.05.047 BindingDB Entry DOI: 10.7270/Q2377BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50178352 (CHEMBL3814457) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of His-tagged full length human recombinant SPHK2 expressed in baculovirus infected fall armyworm Sf9 cell expression system using sphingo... | Bioorg Med Chem 24: 3218-30 (2016) Article DOI: 10.1016/j.bmc.2016.05.047 BindingDB Entry DOI: 10.7270/Q2377BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50393642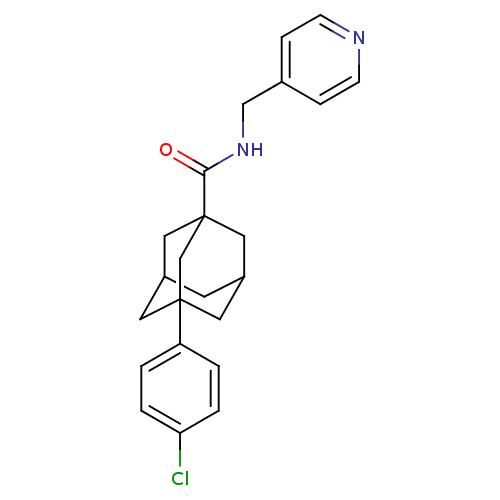 (CHEMBL2158685) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of SPHK2 (unknown origin) | Bioorg Med Chem 24: 3218-30 (2016) Article DOI: 10.1016/j.bmc.2016.05.047 BindingDB Entry DOI: 10.7270/Q2377BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50240721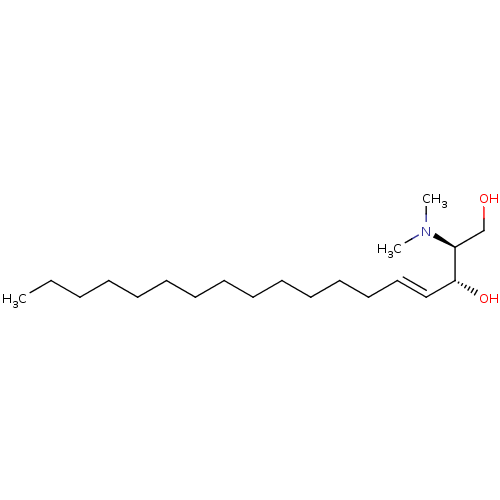 ((E)-(2S,3R)-2-Dimethylamino-octadec-4-ene-1,3-diol...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of His-tagged full length human recombinant SPHK2 expressed in baculovirus infected fall armyworm Sf9 cell expression system using sphingo... | Bioorg Med Chem 24: 3218-30 (2016) Article DOI: 10.1016/j.bmc.2016.05.047 BindingDB Entry DOI: 10.7270/Q2377BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50240721 ((E)-(2S,3R)-2-Dimethylamino-octadec-4-ene-1,3-diol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of His-tagged full length human recombinant SPHK1 expressed in baculovirus infected fall armyworm Sf9 cell expression system using sphingo... | Bioorg Med Chem 24: 3218-30 (2016) Article DOI: 10.1016/j.bmc.2016.05.047 BindingDB Entry DOI: 10.7270/Q2377BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of SPHK1 (unknown origin) | Bioorg Med Chem 24: 3218-30 (2016) Article DOI: 10.1016/j.bmc.2016.05.047 BindingDB Entry DOI: 10.7270/Q2377BN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of SPHK2 (unknown origin) | Bioorg Med Chem 24: 3218-30 (2016) Article DOI: 10.1016/j.bmc.2016.05.047 BindingDB Entry DOI: 10.7270/Q2377BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50591813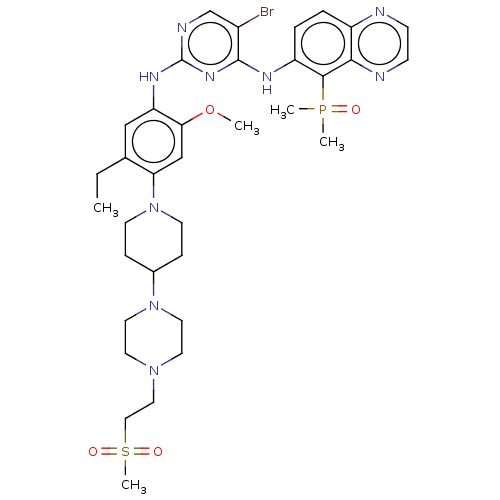 (CHEMBL5206715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128729 BindingDB Entry DOI: 10.7270/Q2930Z4T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50591813 (CHEMBL5206715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128729 BindingDB Entry DOI: 10.7270/Q2930Z4T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50591813 (CHEMBL5206715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128729 BindingDB Entry DOI: 10.7270/Q2930Z4T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50591813 (CHEMBL5206715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128729 BindingDB Entry DOI: 10.7270/Q2930Z4T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50166941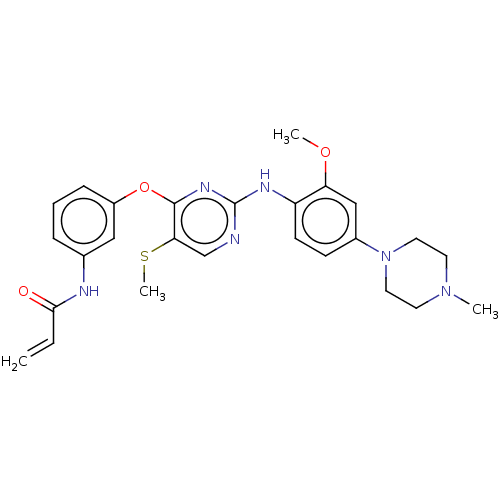 (CHEMBL3797606) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of EGFR T790M/L858R mutant (unknown origin) expressed in baculovirus expression system after 1 hr by ELISA | Bioorg Med Chem 24: 2673-80 (2016) Article DOI: 10.1016/j.bmc.2016.04.032 BindingDB Entry DOI: 10.7270/Q26W9D0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50166942 (CHEMBL3798501) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of EGFR T790M/L858R mutant (unknown origin) expressed in baculovirus expression system after 1 hr by ELISA | Bioorg Med Chem 24: 2673-80 (2016) Article DOI: 10.1016/j.bmc.2016.04.032 BindingDB Entry DOI: 10.7270/Q26W9D0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50185140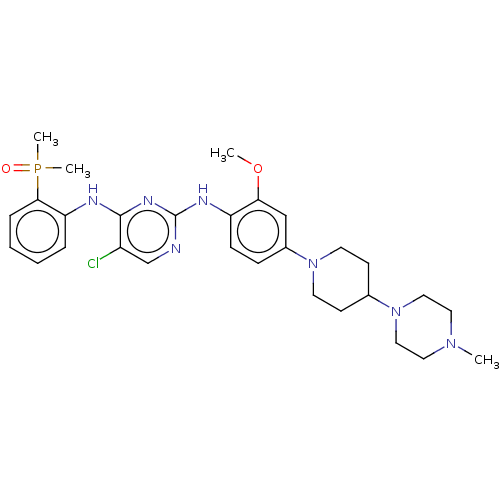 (AP-26113 | Brigatinib | US11248003, Example Brigat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128729 BindingDB Entry DOI: 10.7270/Q2930Z4T | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50591813 (CHEMBL5206715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128729 BindingDB Entry DOI: 10.7270/Q2930Z4T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448442 (US10695347, Compound I-8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448455 (US10695347, Compound I-82) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448447 (US10695347, Compound I-13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448447 (US10695347, Compound I-13) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448448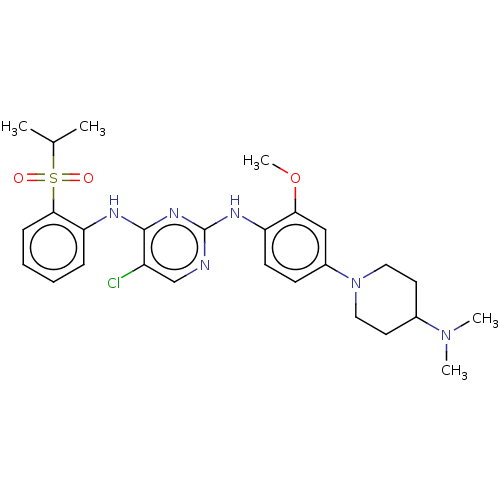 (US10695347, Compound I-14) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448448 (US10695347, Compound I-14) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448442 (US10695347, Compound I-8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448455 (US10695347, Compound I-82) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448450 (US10695347, Compound I-16) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448458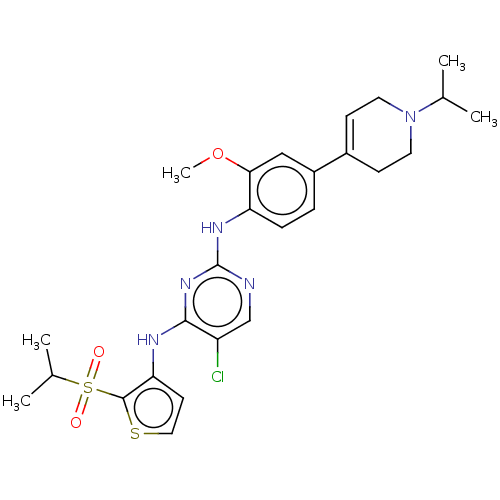 (US10695347, Compound I-86) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448458 (US10695347, Compound I-86) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448461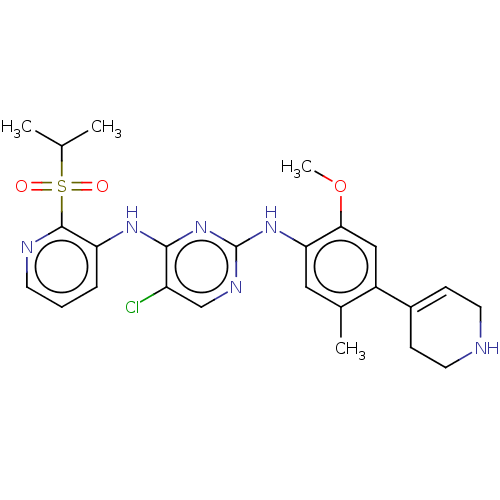 (US10695347, Compound I-89) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448465 (US10695347, Compound I-95) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448465 (US10695347, Compound I-95) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448466 (US10695347, Compound I-98) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448466 (US10695347, Compound I-98) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448468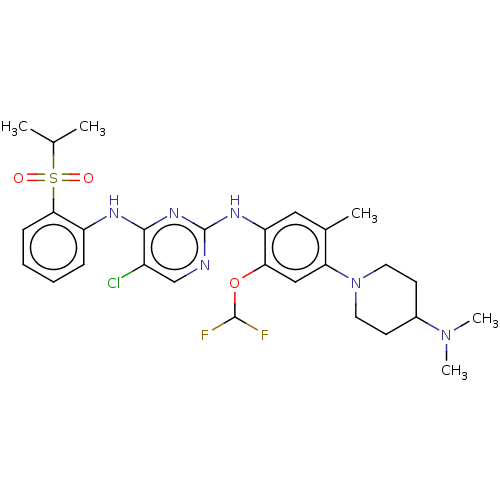 (US10695347, Compound I-102) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448469 (US10695347, Compound I-103) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448470 (US10695347, Compound I-107) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448473 (US10695347, Compound I-111) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448473 (US10695347, Compound I-111) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448474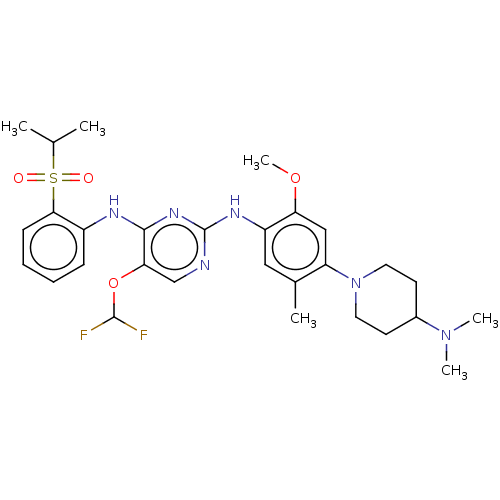 (US10695347, Compound I-112) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448474 (US10695347, Compound I-112) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448475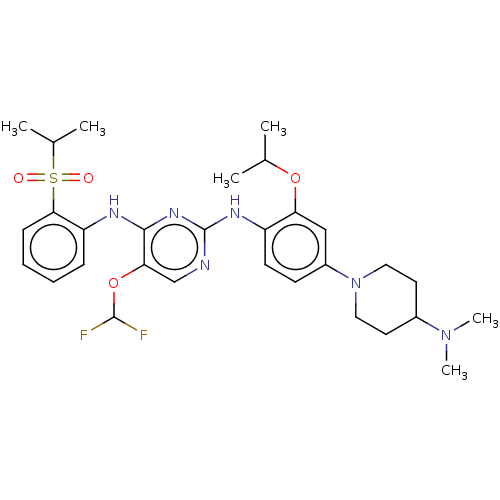 (US10695347, Compound I-113) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448475 (US10695347, Compound I-113) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448476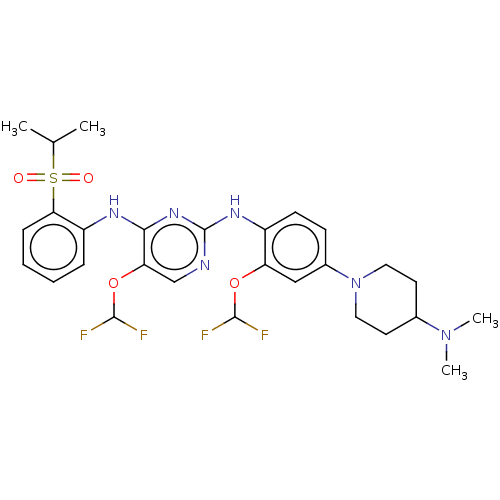 (US10695347, Compound I-114) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448476 (US10695347, Compound I-114) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448477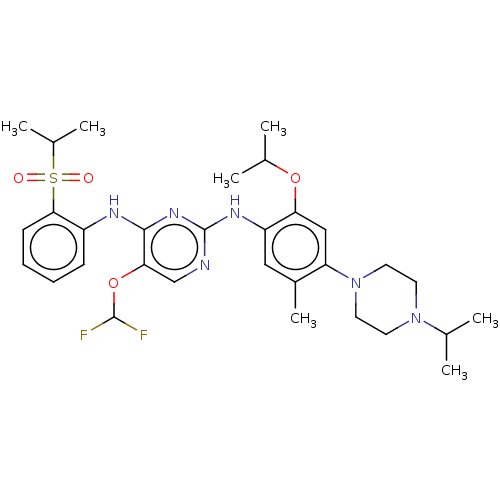 (US10695347, Compound I-115) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM448477 (US10695347, Compound I-115) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439100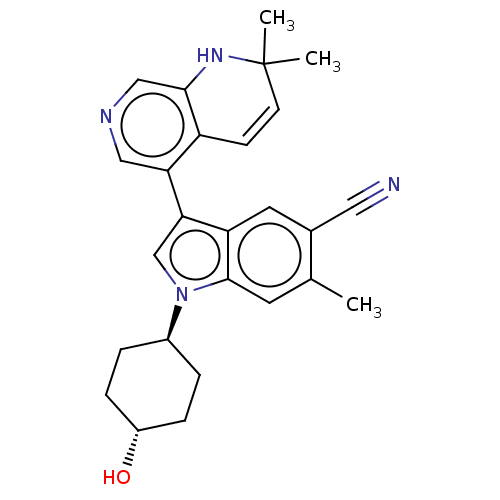 (US10604502, Ex # 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439120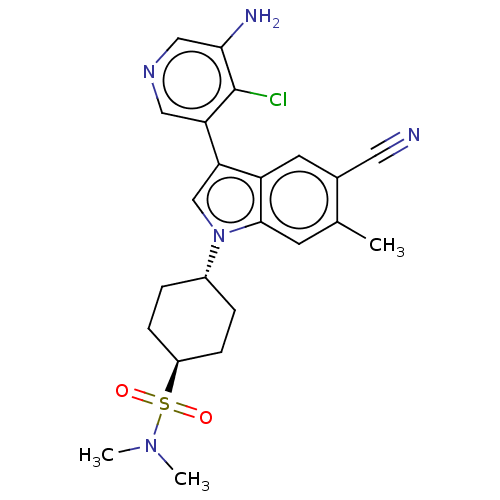 (US10604502, Ex # 108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM448435 (US10695347, Compound I-1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
HUBEI BIO-PHARMACEUTICAL INDUSTRIAL TECHNOLOGICAL INSTITUTE, INC. US Patent | Assay Description 1× Kinase Buffer 50 mM HEPES. pH 7.5 0.0015% Brij-35 10 mM MgCl2 2 mM DTT 2) stop buffer 100 mM HEPES, pH 7... | US Patent US10695347 (2020) BindingDB Entry DOI: 10.7270/Q2M90CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 888 total ) | Next | Last >> |