

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512017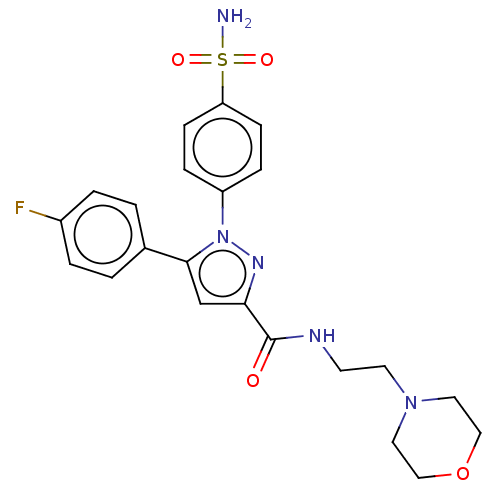 (CHEMBL4552101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512029 (CHEMBL4445481) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512044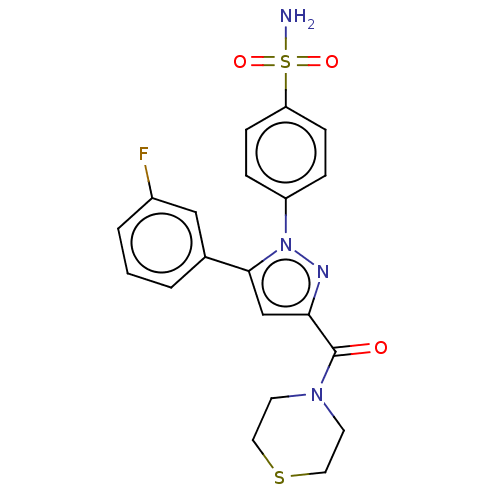 (CHEMBL4529375) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512028 (CHEMBL4462245) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512024 (CHEMBL4476757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512040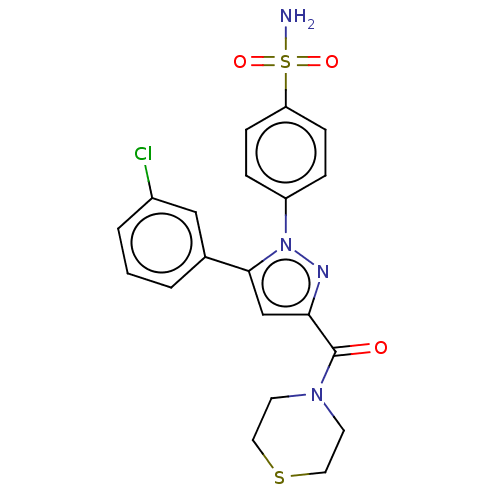 (CHEMBL4440974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512023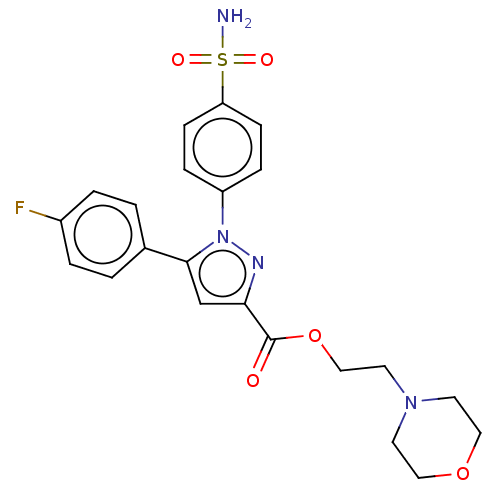 (CHEMBL4439374) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512025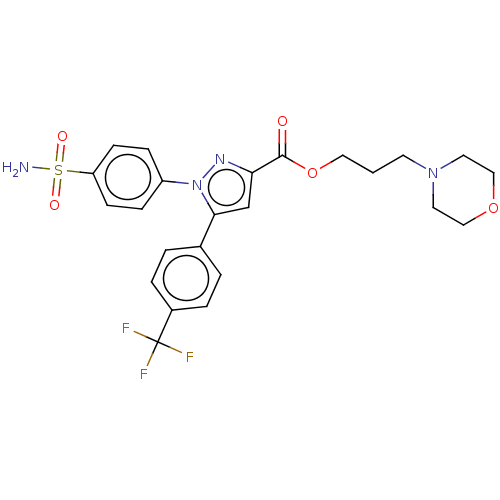 (CHEMBL4451916) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512032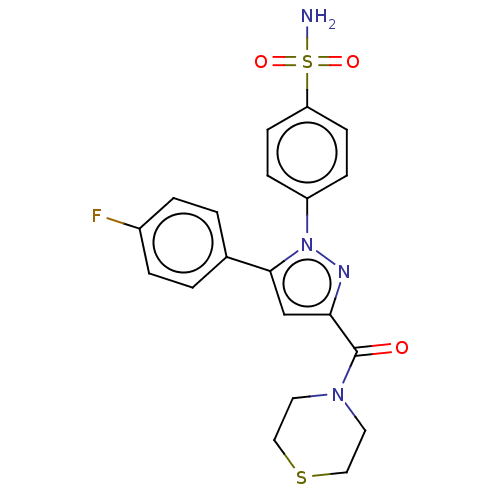 (CHEMBL4443996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512050 (CHEMBL4553947) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512030 (CHEMBL4521969) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512033 (CHEMBL4447638) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512031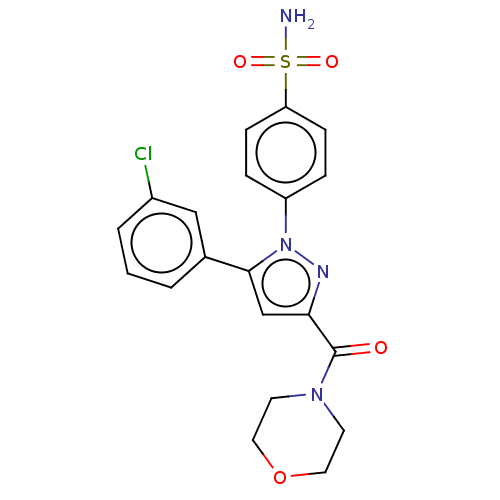 (CHEMBL4532080) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512047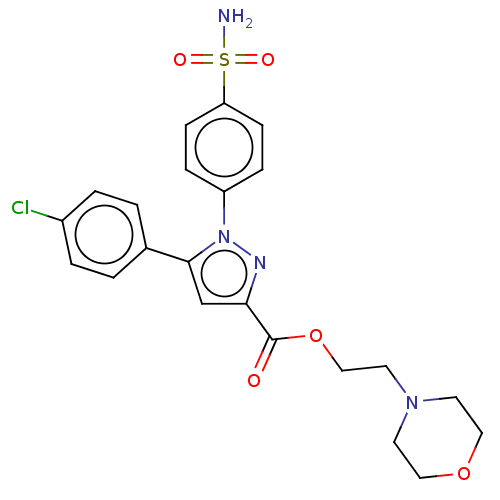 (CHEMBL4530289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512011 (CHEMBL4560235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512035 (CHEMBL4467569) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512045 (CHEMBL4569221) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512041 (CHEMBL4545277) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512020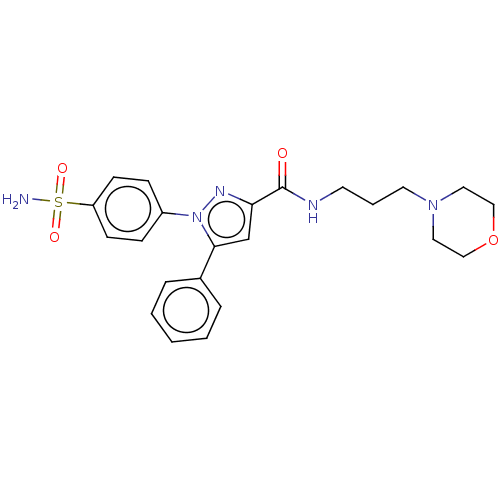 (CHEMBL4438116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512037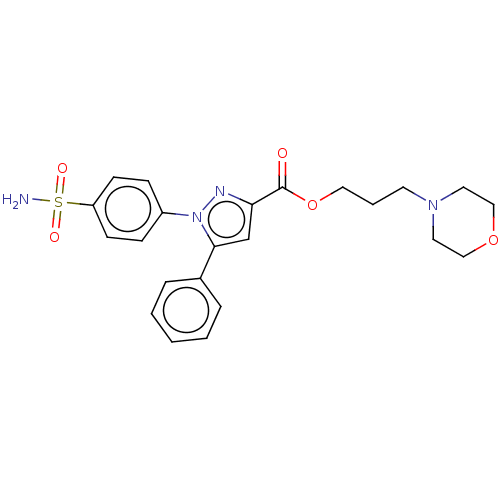 (CHEMBL4473603) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512021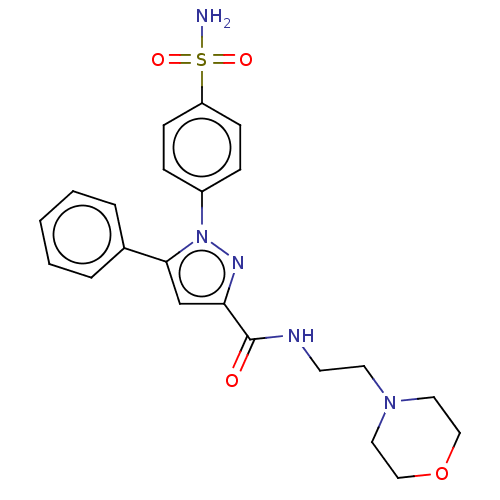 (CHEMBL4540871) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512019 (CHEMBL4559201) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512029 (CHEMBL4445481) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512016 (CHEMBL4458211) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori) | BDBM50467083 (CHEMBL4293995) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori urease after 50 mins by indophenol method | Bioorg Med Chem Lett 28: 3182-3186 (2018) Article DOI: 10.1016/j.bmcl.2018.08.025 BindingDB Entry DOI: 10.7270/Q2PN9894 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512028 (CHEMBL4462245) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512049 (CHEMBL4520676) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512018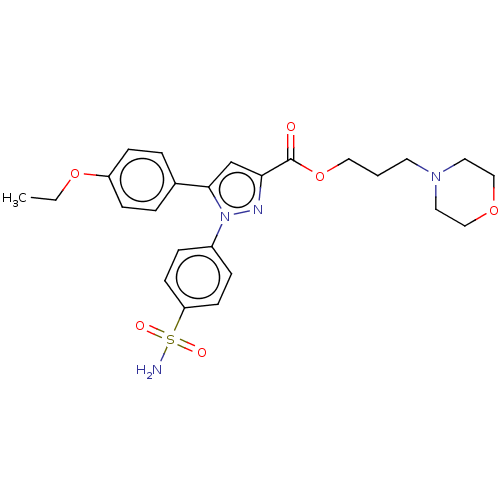 (CHEMBL4569865) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512048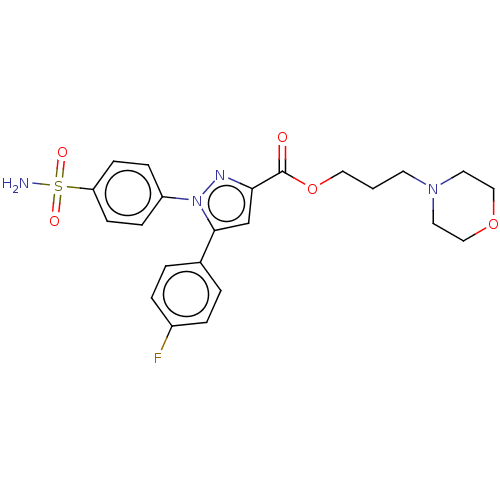 (CHEMBL4475455) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512025 (CHEMBL4451916) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512039 (CHEMBL4441218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512024 (CHEMBL4476757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512037 (CHEMBL4473603) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512023 (CHEMBL4439374) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512027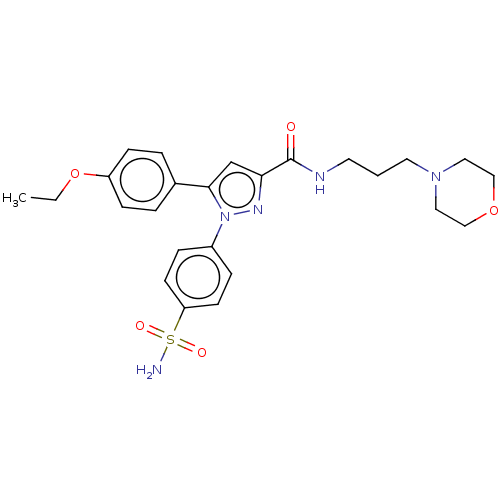 (CHEMBL4455944) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512014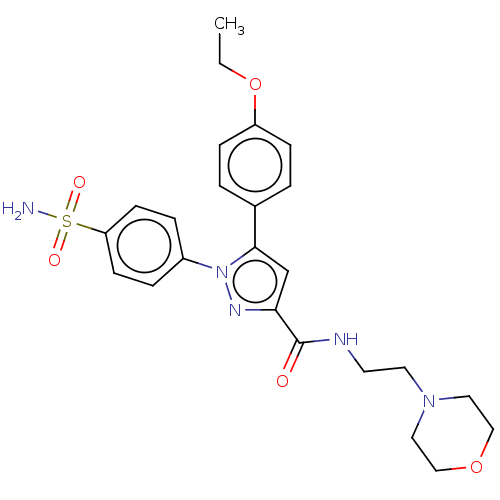 (CHEMBL4532838) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512022 (CHEMBL4460665) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512014 (CHEMBL4532838) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512038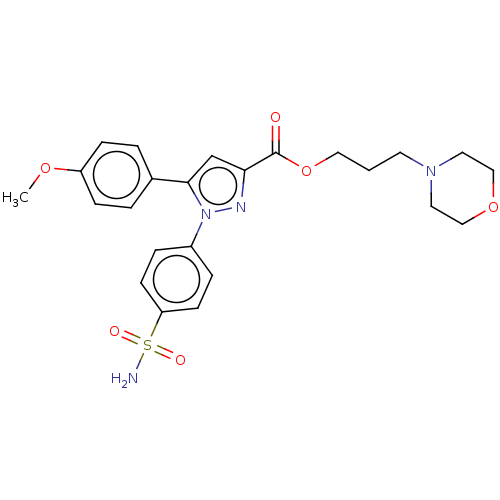 (CHEMBL4553634) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512040 (CHEMBL4440974) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512026 (CHEMBL4440769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512047 (CHEMBL4530289) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512011 (CHEMBL4560235) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512046 (CHEMBL4515463) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512033 (CHEMBL4447638) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512026 (CHEMBL4440769) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512027 (CHEMBL4455944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50512020 (CHEMBL4438116) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat 5-LOX assessed as calcium ionophore A23187-stimulated LTB4 production preincubated for 30 mins followed by calcium i... | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |