

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50543309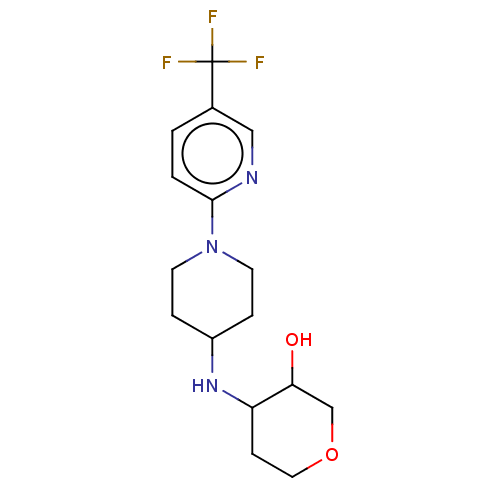 (CHEMBL4647090) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-DTG from recombinant sigma 2 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5 hrs by ... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543309 (CHEMBL4647090) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from recombinant sigma 1 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543307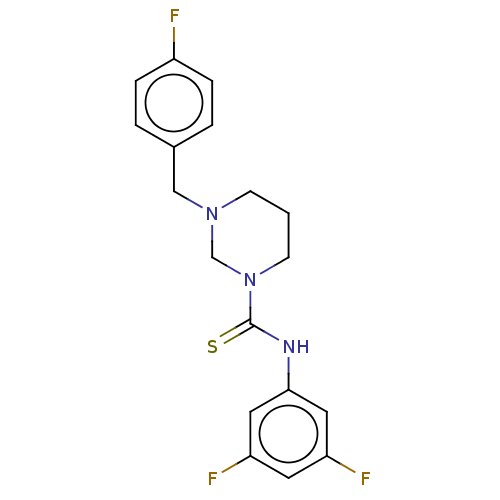 (CHEMBL4644243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from recombinant sigma 1 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543314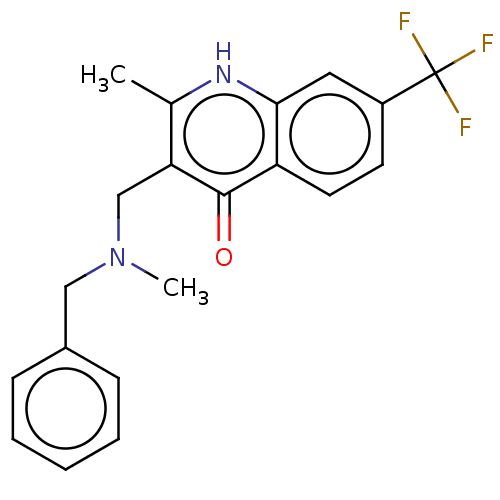 (CHEMBL4648914) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from recombinant sigma 1 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543308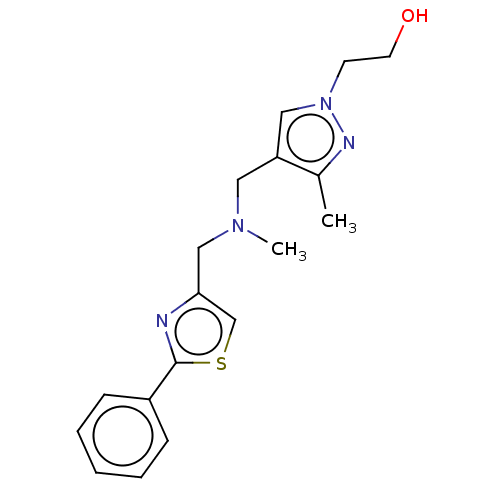 (CHEMBL4640394) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from recombinant sigma 1 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543312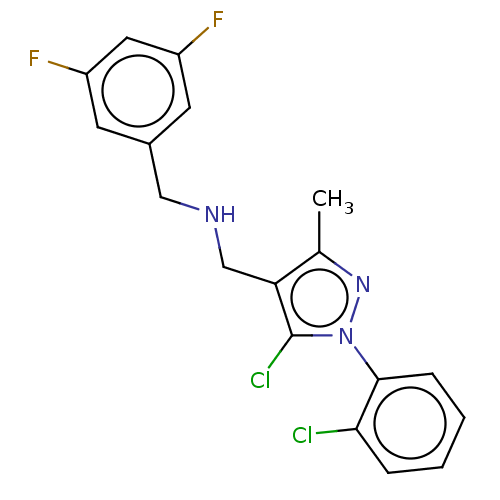 (CHEMBL4648233) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from recombinant sigma 1 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543310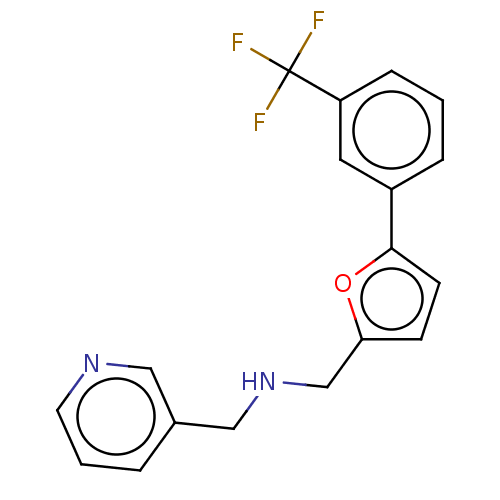 (CHEMBL4640844) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from recombinant sigma 1 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543311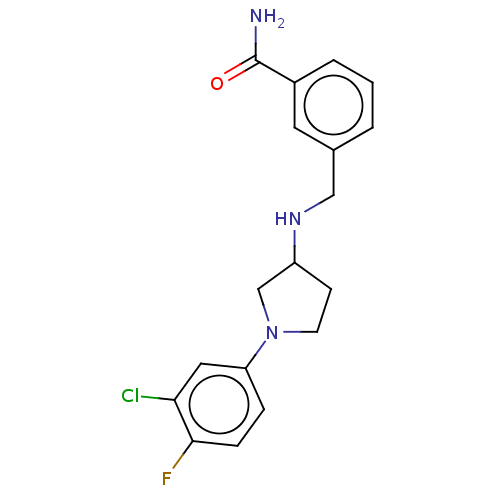 (CHEMBL4641016) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from recombinant sigma 1 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50543311 (CHEMBL4641016) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-DTG from recombinant sigma 2 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5 hrs by ... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543313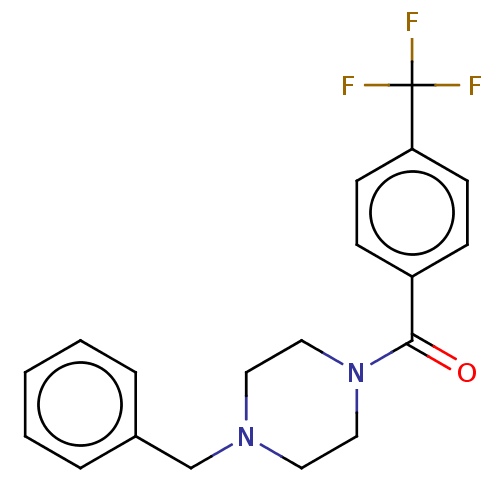 (CHEMBL4644981) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 915 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from recombinant sigma 1 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50543307 (CHEMBL4644243) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 949 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-DTG from recombinant sigma 2 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5 hrs by ... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50543312 (CHEMBL4648233) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 985 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-DTG from recombinant sigma 2 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5 hrs by ... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50543308 (CHEMBL4640394) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-DTG from recombinant sigma 2 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5 hrs by ... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50543314 (CHEMBL4648914) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H](+)-DTG from recombinant sigma 2 receptor (unknown origin) expressed in baculovirus infected Sf9 cell membranes after 1.5 hrs by ... | ACS Med Chem Lett 11: 1555-1561 (2020) Article DOI: 10.1021/acsmedchemlett.9b00314 BindingDB Entry DOI: 10.7270/Q2MK6HGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carnitine O-palmitoyltransferase 1, liver isoform (Rattus norvegicus) | BDBM50033117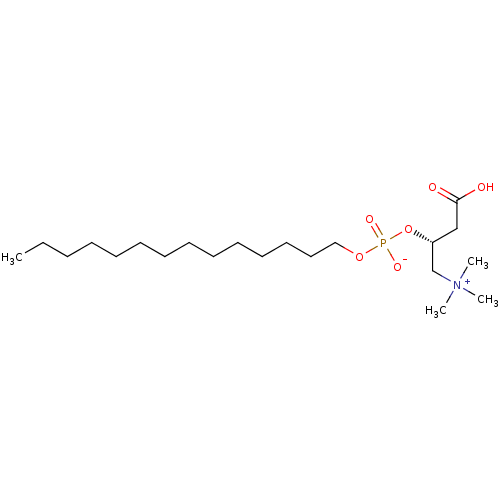 (CHEMBL114113 | [3-Carboxy-2-(hydroxy-tetradecyloxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of carnitine palmitoyltransferase I withrespect to palmitoyl CoA in rat | J Med Chem 38: 3448-50 (1995) BindingDB Entry DOI: 10.7270/Q2VQ31R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239500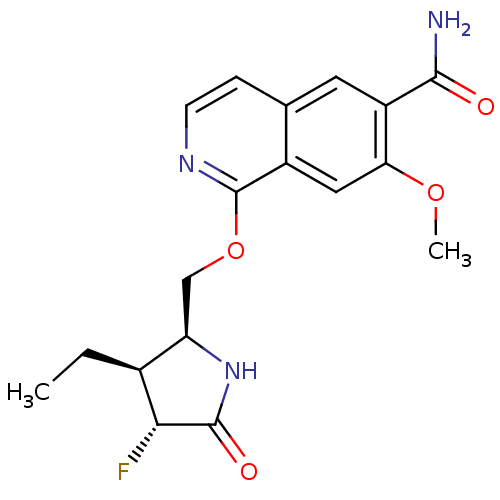 (CHEMBL4066705 | US10329302, Example 337 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403204 (US10329302, Example 360 | US10793579, Example 360 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403214 (US10329302, Example 370 | US10793579, Example 370 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239500 (CHEMBL4066705 | US10329302, Example 337 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239500 (CHEMBL4066705 | US10329302, Example 337 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239500 (CHEMBL4066705 | US10329302, Example 337 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403214 (US10329302, Example 370 | US10793579, Example 370 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403204 (US10329302, Example 360 | US10793579, Example 360 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403214 (US10329302, Example 370 | US10793579, Example 370 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403204 (US10329302, Example 360 | US10793579, Example 360 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403169 (US10329302, Example 323 | US10793579, Example 323 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403189 (US10329302, Example 345 | US10793579, Example 345 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403035 (US10329302, Example 183 | US10793579, Example 183 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403189 (US10329302, Example 345 | US10793579, Example 345 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403169 (US10329302, Example 323 | US10793579, Example 323 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM402999 (US10329302, Example 146 | US10793579, Example 146 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403035 (US10329302, Example 183 | US10793579, Example 183 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403169 (US10329302, Example 323 | US10793579, Example 323 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403189 (US10329302, Example 345 | US10793579, Example 345 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM402999 (US10329302, Example 146 | US10793579, Example 146 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403035 (US10329302, Example 183 | US10793579, Example 183 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM402999 (US10329302, Example 146 | US10793579, Example 146 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 3013 total ) | Next | Last >> |