

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Feline coronavirus (strain FIPV WSU-79/1146) (FCoV...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Feline coronavirus (strain FIPV WSU-79/1146) (FCoV...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189854 (6-bromo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50590329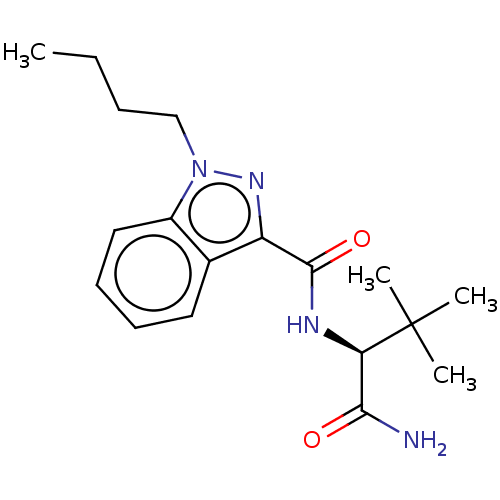 (CHEMBL5169682) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (PEDV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (PEDV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50590329 (CHEMBL5169682) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189841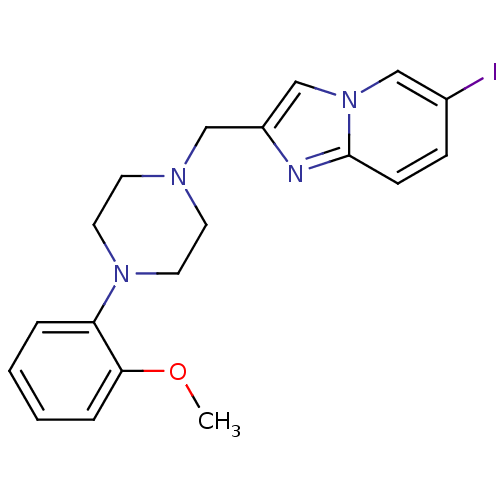 (6-iodo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-OC43) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-OC43) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50094505 (2-((4-phenylpiperazin-1-yl)methyl)-1H-indole-5-car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189852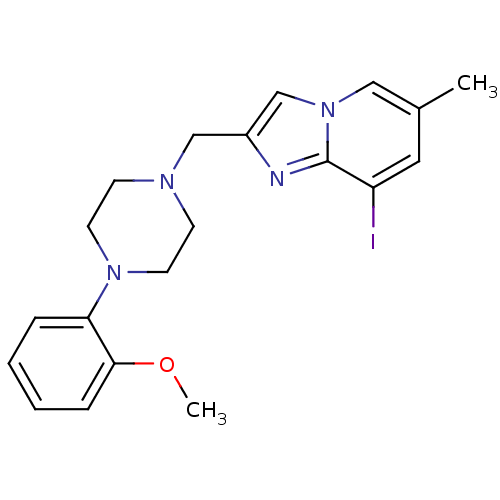 (8-iodo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-NL63) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-NL63) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189843 (2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]-6-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-HKU1) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-HKU1) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50590329 (CHEMBL5169682) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50590329 (CHEMBL5169682) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189844 (2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]-5-(pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189839 (6-bromo-2-[4-(3,4-dichlorophenyl)piperazin-1-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189851 (8-bromo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50094503 (2-((4-(4-fluorophenyl)piperazin-1-yl)methyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (MHV-A59) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (MHV-A59) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50590328 (CHEMBL5180182) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50590328 (CHEMBL5180182) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189856 (6-chloro-2-[4-(3,4-dichlorophenyl)piperazin-1-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189853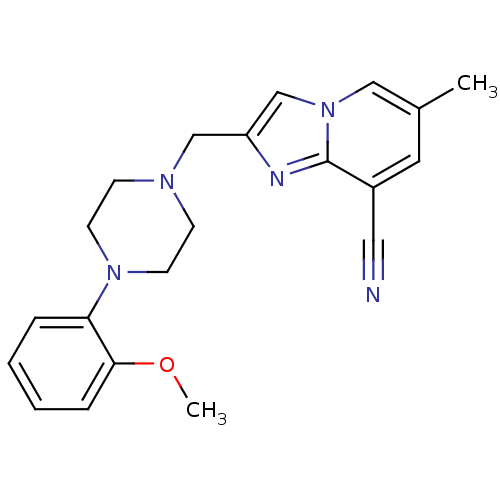 (2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]-6-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50590326 (CHEMBL5202843) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50590326 (CHEMBL5202843) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50590326 (CHEMBL5202843) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50590326 (CHEMBL5202843) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM218837 (TBMB-PK15 (10)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow | Assay Description The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... | Chembiochem 18: 387-395 (2017) Article DOI: 10.1002/cbic.201600612 BindingDB Entry DOI: 10.7270/Q2H9942B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189848 (2-[4-(3,4-dichlorophenyl)piperazin-1-ylmethyl]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-229E) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-229E) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189840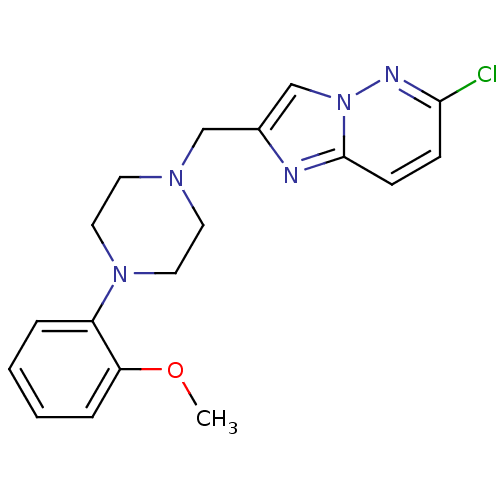 (6-chloro-2-[4-(2-methoxyphenyl)piperazin-1-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50534816 (CHEMBL4591500) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... | J Med Chem 59: 7801-17 (2016) Article DOI: 10.1021/acs.jmedchem.6b00070 BindingDB Entry DOI: 10.7270/Q2697721 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50189847 (2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]-6-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Th�rapeutique Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine receptor D4.4 in CHO cell membrane | J Med Chem 49: 3938-47 (2006) Article DOI: 10.1021/jm060166w BindingDB Entry DOI: 10.7270/Q20G3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 4886 total ) | Next | Last >> |